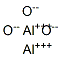Description
Activated alumina is a form of aluminum oxide (Al2O3) with many industrial uses. It continues to grow in importance and its use as a catalyst in the oil and gas industry. It is amorphous or crystalline alumina, which has been partially or completely dehydrated and has a large surface area per unit mass. Activated alumina is made from hydrated alumina, namely, Al2O3.nH2O, where n = 1˜3, by calcining to get n close to 0.5. It is a white or tan-colored material with a chalky appearance.

Uses
Activated alumina is a highly effective adsorbent in both gas and liquid applications and, as such, is employed by several industries for targeted removal of components from other media. As an adsorbent, activated alumina is most well known for its use in water filtration applications, where it serves as a cost-effective adsorbent for removing fluoride from water. It can also remove various other contaminants, including arsenic, lead, and sulfur.
Like an adsorbent, activated alumina can also adsorb water from the air, allowing it to be used as a desiccant; activated alumina can capture and trap water to keep things dry, much like silica gel. As a desiccant, it can adsorb up to 20% of its weight in water at a relative humidity of 50%. Activated alumina is employed as a desiccant in a wide variety of applications, including the removal of water vapor from gases in industrial settings. Water adsorbed onto it can then be desorbed via thermal treatment, and the alumina reused.
Activated alumina is also widely used as a catalyst, with roles as the catalyst itself and an inert carrier or substrate for other catalysts. As a catalyst, activated alumina is known for its role as a Claus catalyst; activated alumina is the most commonly used Claus catalyst in sulfur recovery endeavors at oil and gas refineries.
Production Methods
Activated alumina is manufactured by dehydroxylation of aluminum oxide hydroxide (Boehmite), which produces a highly porous substance with a surface area typically 150–380 m2 per gram. The term “activated alumina” refers to the activation due to calcination. The pore structure is resistant to high temperatures and abrasion, which is why activated alumina is used as a thermochemical catalyst. Both Brönsted and Lewis acid sites are contained in activated alumina catalysts, which are required for catalytic cracking.
Advantages
Activated alumina exhibits several characteristics that make it ideal in many industrial process settings. This includes a high crush strength, resistance to thermal shock, resistance to chemical attack, and more. The characteristic that has pushed activated alumina to the forefront of many applications is its capability as an adsorbent, courtesy of its high porosity and surface area.