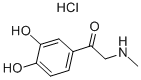
Epinephrine
- Product NameEpinephrine
- CAS51-43-4
- CBNumberCB00131044
-
MFC9H13NO3
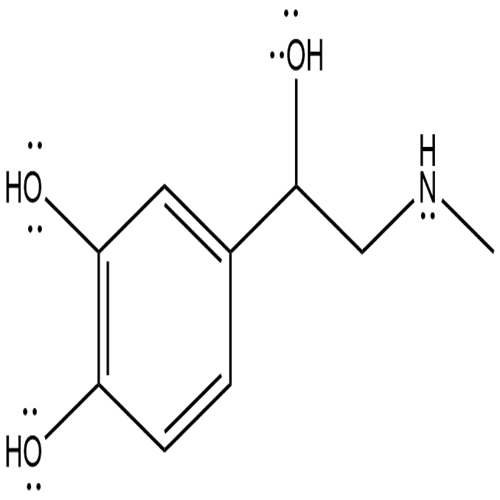
Lewis structure
- MW183.2
- EINECS200-098-7
- MDL NumberMFCD00002204
- MOL File51-43-4.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 215 °C (dec.)(lit.) |
| Boiling point | 316.88°C (rough estimate) |
| alpha | -51.5 º (c=4, 1M HCl, dry sub) |
| Density | 1.1967 (rough estimate) |
| refractive index | -51.5 ° (C=4, 1mol/L HCl) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, in ethanol (96 per cent) and in methylene chloride. It dissolves in hydrochloric acid. |
| form | Fine Crystalline Powder |
| pka | 8.66(at 25℃) |
| color | White to light beige |
| optical activity | -53.222~25 (c 1.2, 0.5 mol dm-3 HCl)-50.617 (c 7.5, 0.37 mol dm-3 HCl), L +50.5 (HCl) |
| Water Solubility | <0.01 g/100 mL at 18 ºC |
| Sensitive | Air & Light Sensitive |
| Decomposition | > 212°C |
| Merck | 14,3619 |
| BRN | 2368277 |
| Stability | Stable. Incompatible with acids, acid chlorides, acid anhydrides, oxidizing agents. Light sensitive. |
| CAS DataBase Reference | 51-43-4(CAS DataBase Reference) |
| FDA 21 CFR | 341.16 |
| EWG's Food Scores | 1-3 |
| NCI Dictionary of Cancer Terms | adrenaline; epinephrine |
| FDA UNII | YKH834O4BH |
| ATC code | A01AD01,B02BC09,C01CA24,R01AA14,R03AA01,S01EA01,S01EA51 |
| NIST Chemistry Reference | L-adrenaline(51-43-4) |
| EPA Substance Registry System | Epinephrine (51-43-4) |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H301+H331-H310 | |||||||||
| Precautionary statements | P261-P262-P280-P301+P310-P302+P352+P310-P304+P340+P311 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 23/24/25-52/53-36/37/38-33 | |||||||||
| Safety Statements | 36/37/39-45-61-26-23 | |||||||||
| RIDADR | UN 2811 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DO2625000 | |||||||||
| F | 8-10-23 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29373100 | |||||||||
| Hazardous Substances Data | 51-43-4(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 i.p. in mice: 4 mg/kg (Lands) | |||||||||
| NFPA 704: |
|
Epinephrine Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| Sigma-Aldrich E4250 | 1g | $28.9 | (?)-Epinephrine
|
Buy |
| Sigma-Aldrich E4250 | 5g | $98.5 | (?)-Epinephrine
|
Buy |
| Alfa Aesar L04911 | 5g | $83.5 | L-Adrenaline, 98+% |
Buy |
| Alfa Aesar L04911 | 25g | $304 | L-Adrenaline, 98+% |
Buy |
| Cayman Chemical 18626 | 5g | $68 | (-)-Epinephrine ≥95% |
Buy |
Epinephrine Chemical Properties,Usage,Production
Overview
Epinephrine is used for the emergency treatment of severe allergic reactions (including anaphylaxis) to insect bites or stings, medicines, foods, or other substances. It can also be used in the treatment of idiopathic anaphylaxis (unknown cause) or exercise-induced anaphylaxis. In addition to the above function, epinephrine is the primary drug administered during cardiopulmonary resuscitation (CPR) to reverse cardiac arrest.Epinephrine is one of two catecholamines that are produced in the medullary region of the adrenal gland. The effects of epinephrine are ubiquitous; there is scarcely a physiological system that is unaffected by this agent. In general, the effects of epinephrine are associated with a “fight or flight” response that prepares an organism to cope with an emergency.[1, 2] Under conditions of stress and excitement, the secretion of epinephrine is markedly increased. During such situations, adrenomedullary discharge causes a redistribution of blood flow toward the working muscles, an in-crease in cardiac output and blood pressure, arousal of the central nervous system, and mobilization of large energy stores [3].
Epinephrine's first use in cardiac arrest has frequently been ascribed to a
1906 work by Crile and Dolley, in which they demonstrated that, compared with artificial ventilation and cardiac massage alone, the infusion of epinephrine greatly increased the proportion of dogs that could be resuscitated successfully[4]. However, this work was antedated by Crile's 1903 and 1904 reports on successful resuscitation of animals "apparently dead" for up to 15 minutes, using "artificial respiration, rhythmic pressure upon the heart, and the infusion of adrenalin[5-7]. Of interest is his mention of the successful temporary resuscitation of two human beings using these three techniques[8].
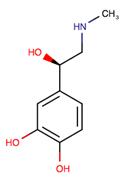
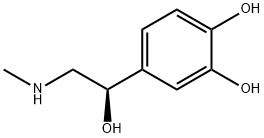
Figure 1 the chemical structure of epinephrine;
Indication
Epinephrine or related sympathomimetic agents are typically prescribed for acute and chronic treatment of clinical disorders such as hypotension, anaphylactic and allergic reactions, and bronchial asthma. 91 Its cardiac effects may be of use in restoring cardiac rhythm in cardiac arrest due to various causes, but is not used in cardiac failure or in hemorrhagic, traumatic, or cardiogenic shock.[3, 4]Epinephrine injection is indicated in the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging insects (e.g., order Hymenoptera, which include bees, wasps, hornets, yellow jackets and fire ants) and biting insects (e.g., triatoma, mosquitos), allergen immunotherapy, foods, drugs, diagnostic testing substances (e.g., radiocontrast media) and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis. Injectable epinephrine is intended for immediate/urgent administration in patients, who are found to be at increased risk for anaphylaxis, including individuals with a history of anaphylaxis.
Pharmacology and Mode of action
During exercise, elevated levels of catecholamines, particularly epinephrine, have important effects on skeletal muscle function, including increased glycogen breakdown, glucose uptake, and increased oxygen consumption[12-15]. Epinephrine also exerts a positive inotropic effect on fast-contracting skeletal muscles.Epinephrine exerts its effect on alpha and beta-adrenergic receptors[16-18]. Drugs bind to specific cell surface receptors, and the receptor binding properties of a drug determine its individual pharmacological effects. In the case of adrenergic drug, it was suggested that there are two types of receptors: alpha and beta-adrenergic receptors. Stimulation of alphareceptors caused certain physiological effects, while stimulation of beta-receptors caused other, or opposite, effects. For instance, alpha-receptor stimulation caused vasoconstriction, while beta-stimulation caused vasodilatation.
Through its action on alpha-adrenergic receptors, epinephrine minimizes the vasodilation and increased the vascular permeability that occurs during anaphylaxis, which can cause the loss of intravascular fluid volume as well as hypotension. Through its action on beta-adrenergic receptors, epinephrine leads to bronchial smooth muscle relaxation that helps to relieve bronchospasm, wheezing, and dyspnea that may occur during anaphylaxis. Epinephrine also alleviates pruritus, urticaria, and angioedema and may work in relieving gastrointestinal and genitourinary symptoms associated with anaphylaxis because of its relaxing effects on the smooth muscle of the stomach, intestine, uterus, and urinary bladder.
Dosage and administration
The optimal dosage of epinephrine in cardiac arrest is not known.1l Investigators working in animal models have used dosages greater, on a per-weight basis, than those currently recommended for human beings. Recent studies in animals and human beings have specifically evaluated the effects of higher epinephrine doses and suggest that standard doses may be too low[19, 20].For its injection form when applied subcutaneously or intramuscularly 0.2 to 1 mL (mg). Start with a small dose and increase if required. The subcutaneous is the preferred route of administration. If given intramuscularly, injection into the buttocks should be avoided. For the treatment of bronchial asthma and certain allergic manifestations, e.g., angioedema, urticaria, serum sickness, anaphylactic shock, you should administrate epinephrine subcutaneously. For bronchial asthma in pediatric patients, administer 0.01 mg/kg or 0.3 mg/m2 to a maximum of 0.5 mg subcutaneously, repeated every four hours if required.
For the treatment of cardiac resuscitation – apply a dose of 0.5 mL (0.5 mg) diluted to 10 mL with sodium chloride injection and administer intravenously or intracardially to restore myocardial contractility. External cardiac massage should follow intracardial administration to permit the drug to enter coronary circulation. Parenteral drug products should be inspected visually for particulate matter and discoloration whenever solution and container permit.
Pharmacodynamics
Following I.V. (intravenous) injection, epinephrine disappears rapidly from the blood stream. Subcutaneously or I.M. (intramuscular) administered epinephrine has a rapid onset and short duration of action. Subcutaneous (SC) administration during asthmatic attacks may produce bronchodilation within 5 to 10 minutes, and maximal effects may occur within 20 minutes. The drug becomes fixed in the tissues rapidly.Epinephrine is rapidly inactivated mainly by enzymic transformation to metanephrine or normetanephrine, either of which is then conjugated and excreted in the urine in the form of both sulfates and glucuronides. Either sequence results in the formation of 3-methoxy-4hydroxy-mandelic acid (vanillylmandelic acid, VMA) that is detectable in the urine. Epinephrine is rapidly inactivated in the body mostly by the enzymes COMT (catechol-O-methyltransferase) and MAO (monoamine oxidase). The liver is rich in the above enzymes, and is a primary, although not essential, tissue in the degradation process. The majority of the dose of epinephrine is accounted for by excretion of metabolites in the urine. The plasma half-life is approximately 2-3 minutes. However, when administered by subcutaneous or intramuscular injection, local vasoconstriction may delay absorption so that epinephrine's effects may last longer than the half-life suggests[21, 22].
Precaution and contradiction
Epinephrine should be contraindicated in patients suffering one or several of the following conditions: narrow angle (congestive) glaucoma, shock, during general anesthesia with halogenated hydrocarbons or cyclopropane and in individuals with organic brain damage. Epinephrine should also be contraindicated in cases of local anesthesia of certain areas, e.g., fingers, toes due to the danger of vasoconstriction producing sloughing of tissue; in labor because it may delay the second stage; in cardiac dilatation and coronary insufficiency. Epinephrine is also not allowed in those cases where vasopressor drugs may be contraindicated, e.g., in thyrotoxicosis, diabetes, in obstetrics when maternal blood pressure is in excess of 130/80, and in hypertension and other cardiovascular disorders.Epinephrine injection should be protected from exposure to light. Do not remove vial from carton until ready to use. Stop using the solution in cases of it becomes pinkish or darker than slightly yellow or if it contains a precipitate. Epinephrine can be readily destroyed by alkalies and oxidizing agents. In the latter category are oxygen, chlorine, bromine, iodine, permanganates, chromates, nitrites, and salts of easily reducible metals, especially iron. Epinephrine should be administered with caution to infants and children. Syncope has occurred following the administration of epinephrine to asthmatic children. Having an allergic reaction while pregnant or nursing could harm both mother and baby. You may need to use epinephrine during pregnancy or while you are breast-feeding. Seek emergency medical attention right away after using the injection. In an emergency, you may not be able to tell caregivers if you are pregnant or breast-feeding. Make sure any doctor caring for your pregnancy or your baby knows you received this medicine. You should tell your doctor if you have a history of heart disease or high blood pressure, asthma, Parkinson’s disease, depression or mental illness, a thyroid disorder or diabetes.
Adverse reactions
Transient and minor side effects include anxiety, headache, fear, and palpitations often occur with therapeutic doses[22]. The frequency is higher in hyperthyroid individuals. Repeated local injections can result in necrosis at sites of injection from vascular constriction. “Epinephrine-fastness” can occur with prolonged use.Repeated local injections can lead to necrosis at sites of injection from vascular constriction[22]. Systemic effect may include cerebral hemorrhage; weakness; dizziness; pallor; respiratory difficulty; hemiplegia; apprehensiveness; sweating; nausea; subarachnoid hemorrhage; anginal pain in patients with angina pectoris; anxiety; restlessness; throbbing headache; tremor; palpitation; vomiting[22].
Patients in spontaneous circulation accidentally given large doses of epinephrine immediately complain of chest or abdominal pain and show signs of excessive sympathetic stimulation with severe transient hypertension, often followed by pulmonary edema and hypotension. An IV dose of 3 mg has been reported as fatal, while other patients have survived IV doses as large as 30 mg[23-25].
References
- Cannon WB, Nice LB: The effect of splanchnic stimulation on muscular fatigue. Am J Physiol 191 1; 29:24-25.
- Cannon WB, Nice LB: The effect of adrenaline secretion on muscular fatigue. Am J Phyiol 1912: 32:44-60.
- Tepperrnan J, Tepperman HM: Metabolic and Endocrine Physiology Chicago, Yearbook Medical Publishers, 1987. pp 229-245.
- Crile GW, Dolley DH: An experimental research into the resuscitation of dogs killed by anesthetics and asphyxia. J Exp Med 1906;8:713-724.
- Crile GW: Preliminary note on a method of resuscitation of apparently recently dead animals. Cleve Med 1903;2:35.
- Crile GW: Resuscitation of animals apparently dead (letter). Indian Lancet 1904;23:913.
- Crile GW: Resuscitation of animals apparently dead. St Louis Med Surg 1903;84:299-302.
- Crile GW: The resuscitation of the apparently dead and a demonstration of the pneumatic rubber suit as a means of controlling blood pressure. Tr South Surg & Gynec Assoc 1904;16: 362-370.
- U’einer N: Norepinephrine, epinephrine, and the sympathomimetic amines, in Gilman AG, Goodman LS, Rall TW, Murad F (Eds): The Pharmacolopcal Basrs of Thpraputzcs. New York, MacMillan, 1985, pp 145180
- Otto CW, Yakaitis RW, Blitt CD: Mechanism of action of epinephrine in resuscitation from asphyxial arrest. Crit Care Med. 1981 Apr; 9(4): 321-4.
- Callaway CW: Epinephrine for cardiac arrest. Curr Opin Cardiol. 2013 Jan; 28(1): 36-42.
- Gonzales ER, Omato JP, Garnett AR, et ah Dose-dependent vasopressor response to epinephrine during CPR in human beings. Ann Emerg Med 1989; 18: 920-926.
- Koehler RC, Michael JR, Guerci AD, et al: Beneficial effect of epinephrine infusion on cerebral and myocardial blood flows during CPR. Ann Ernerg Med 1985; 14: 744-749.
- Joyce SM, Barsan WG, Doan LA: Use of phenylephrine in resuscitation from asphyxial arrest. Ann Emerg Med 1983;12:418-421.
- Brown CG, Werinan HA, Davis EA, etal: The effect of high-dose phenylephrine versus epinephrine on cerebral blood flow during CPR. Ann Emerg Med 1987; 16:743 748.
- Lands AM, Arnold A, McAuliff JP, et ah Differentiation of receptor systems activated by sympathomimetic amines. Nature 1967; 214: 597-598
- Starke K, Taube HD: Relative preand postsynaptic potencies of alpha-adrenoceptor agonists in the rabbit pulmonary artery. Naunyn Schmiedeberg's Arch Pharmacol 1975;291:55-78.
- ArnoldA: Sympathomimetic amine-induced responses of effectors organs subserved by alpha, beta-l, and beta-2 adrenoreceptors, in Szkekeres L (ed): Adrenergic Activators and Inhibitors, Hand book of Experimental Pharmacrdogg Heidelberg, Springer-Verlag, 1980.
- Ralston SH, Babbs CF: Joseph S: Redding's contributions to cardiac resuscitation. Am J Emerg Med 1985;3:247-251.
- Callaham ML: Advances in the management of cardiac arrest. West J Med 1986;145:670-675.
- https://www.medicines.org.uk/emc/product/4289/smpc#PHARMACOKINETIC_PROPS
- https://s3-us-west-.amazonaws.com/drugbank/cite_this/attachments/files/000/001/247/original/Epinephrine.pdf?1534883635
- Novey HS, Meleyco LN: Alarming reaction after intravenous administration of 30 ml of epinephrine. JAMA 1969;207:2435-2436.
- Freedman BJ: Accidental adrenaline overdosage and its treatment with piperoxan. Lancet 1955;2:575-578.
- Levine RD, Orkin LR: Epinephrine overdose; a continuing problem. NY State ] Med 1981;81:1669-1670.
Description
The combination of the catechol nucleus, the β-hydroxyl group, and the N-methyl give EPI a direct action and a strong affinity for all adrenergic receptors. Epinephrine and all other catechols are chemically susceptible to oxygen and other oxidizing agents, especially in the presence of bases and light, quickly decomposing to inactive quinones. Therefore, all catechol drugs are stabilized with antioxidants and dispensed in air-tight amber containers.Chemical Properties
off-white powderOriginator
Adrenalin,Sopharma,BulgariaUses
L-Adrenaline (Epinephrine) belongs to a group of the compounds known as catecholamines, which play an important role in the regulation of physiological process in living organisms. The antioxidant activity and antioxidant mechanism of L-adrenaline was claUses
Endogenous catcholamine with combined α-and β-agonist activity. Principal sympathomimetic hormone produced by the adrenal medulla. Bronchodilator; cardiostimulant; mydriatic; antiglaucoma.Uses
Gibberellic Acid-3 (GA-3) is a plant growth regulatorIndications
Epinephrine administered subcutaneously is used to manage severe acute episodes of bronchospasm and status asthmaticus. In addition to its bronchodilator activity through β-adrenoceptor stimulation, a portion of the therapeutic utility of epinephrine in these acute settings may be due to a reduction in pulmonary edema as a result of pulmonary vasoconstriction, the latter effect resulting from α-adrenoceptor stimulation.Definition
ChEBI: The R-enantiomer of adrenaline. It is a hormone secreted by the adrenal glands resulting in the 'fight-or-flight' response.Production Methods
Epinephrine is synthesized in the body from the nonessential amino acid tyrosine. Tyrosineundergoes hydroxylation to produce DOPA (3,4-dihydroxyphenylalanine). DOPA decarboxylationproduces dopamine, which is hydroxylated to norepinephrine. Norepinephrine, whichis closely related to epinephrine, performs a number of similar functions in the body. The prefix “nor” associated with a compound is used to denote an alkylated nitrogen in the compoundthat has lost an alkyl group. It comes from the German N-ohne-radical, which means Nitrogenwithout the radical. Therefore norepinephrine is epinephrine minus the methyl, CH3, radicalon the nitrogen. The methylation of norepinephrine gives epinephrine.Manufacturing Process
1 part by weight of ω-chloro-3,4-dihydroxyacteophenone in 1 part by weight of ethanol was heated with 60% aqueous solution of methylamine. The crystal of 3,4-dihydroxy-ω-methylaminoacteophenone obtained was transformed in hydrochloride by action of diluted hydrochloric acid. The base of (-)-3,4- dihydroxy-ω-methylaminoacteophenone was prepared by addition of ammonium hydroxide solution.brand name
Bronkaid (Bayer); Epipen (Meridian); Primatene (Wyeth); Sus-Phrine (Forest); Twinject (Verus);Adrefil;Adrehinal;Adrenalin medihal;Adrenalina ace.p.d.;Adrenalina clorhi;Adrenalina delta;Adrenalina fustery;Adrenalina hormona;Adrenalina p davis;Adrenalina wiener;Bronkaid mistometer;Cetanest;D epinefrin;Dento-caine;Depinefrin;Dysne-inhal;E-caprine;Epiboran ofteno;Epinephrine pediatric;Epineramine;Glaucadrine;Glaucoaicon;Glauconin;Glaucotahil;Isopto epinefrina;Levoreninl-adrenaline;Licothionil;Lidoacton;Lyodrin;Marcaom;Methylaminoethanolcatechol;Neo-rybarex;Niphridine;Orostat;P2e1;Paranephrine;Piladren;Sedo-asmol;Suprarenine;Suprexon 5;Susphrine;Vaponephrine;Xylestesin a.Therapeutic Function
VasoconstrictorWorld Health Organization (WHO)
Epinephrine, first isolated in 1899, is the main hormone secreted by the adrenal medulla. It is widely used as a vasoconstrictor substance and in the treatment of anaphylactic shock. Its use in combination with local anaesthetics to prolong infiltration anaesthesia has been associated with systemic reactions including serious cardiovascular and cerebrovascular incidents. Regulations restricting the concentrations permitted in such preparations have been introduced in many countries but combination products containing epinephrine or levarterenol in concentrations of 1:80,000 or less remain widely available. Representative preparations are included in the WHO Model List of Essential Drugs. (Reference: (WHTAC1) The Use of Essential Drugs, 2nd Report of the WHO Expert Committee, 722, , 1985)Biological Functions
Epinephrine is found only in very low concentrations in the mammalian CNS, and it is unlikely to play a major role as a neurotransmitter.General Description
Epinephrine (E, Adrenalin) differs from NE only bythe addition of an N-methyl group. Like the other CAs, E islight sensitive and easily oxidized on exposure to air becauseof the catechol ring system. The development of apink-to-brown color indicates oxidative breakdown. Tominimize oxidation, solutions of the drug are stabilized bythe addition of reducing agents such as sodium bisulfite. Eis also destroyed readily in alkaline solutions and by metals(e.g., Cu, Fe, Zn) and weak oxidizing agents. It is used inaqueous solution for inhalation as the free amine. Like otheramines, it forms salts with acids, hydrochloride, and thebitartrate being the most common.Like NE, it lacks oral activity and has short DOA.However, it is much more widely used clinically than NE.E is a potent stimulant of all α1-,α2-,β1-,β2-, and β3-adrenoceptors, and thus it switches on all possible adrenergicreceptors, leading to a whole range of desired and sideeffects. Particularly prominent are the actions on the heartand on vascular and other smooth muscle. It is a very potentvasoconstrictor and cardiac stimulant. NE has, in general,greater β-activity caused by an additional N-methyl group.Therefore, E is used to stimulate the heart in cardiac arrest.Although intravenous infusion of E has pronounced effectson the cardiovascular system, its use in the treatment ofheart block or circulatory collapse is limited because of itstendency to induce cardiac arrhythmias.
General Description
White to nearly-white microcrystalline powder or granules. Odorless. Melting point 211-212°C. Aqueous solutions are slightly alkaline. Slightly bitter, numbing taste.Air & Water Reactions
L(-)-Epinephrine darkens slowly on exposure to air and light. Water insoluble. Readily soluble in aqueous solutions of inorganic acids. Solutions undergo oxidation in the presence of oxygen.Reactivity Profile
L(-)-Epinephrine is incompatible with oxidizers, alkalis, copper, iron, silver, zinc and other metals; gum and tannin. L(-)-Epinephrine is also incompatible with acids, acid chlorides and acid anhydrides. L(-)-Epinephrine reacts with salts of sulfurous acid .Fire Hazard
Flash point data for L(-)-Epinephrine are not available. L(-)-Epinephrine is probably combustible.Biochem/physiol Actions
Adrenoceptor agonist.Mechanism of action
The effects on pulmonary function are quite rapid, with peak effects occurring within 5 to 15 minutes. Measurable improvement in pulmonary function is maintained for up to 4 hours.The characteristic cardiovascular effects seen at therapeutic doses of epinephrine include increased heart rate, increased cardiac output, increased stroke volume, an elevation of systolic pressure and decrease in diastolic pressure, and a decrease in systemic vascular resistance. The cardiovascular response to epinephrine represents the algebraic sum of both α- and β-adrenoceptor stimulation. A decrease in diastolic blood pressure and a decrease in systemic vascular resistance are reflections of vasodilation, a β2-adrenoceptor response. The increase in heart rate and systolic pressure is the result of either a direct effect of epinephrine on the myocardium, primarily a β1 effect, or a reflex action provoked by a decrease in peripheral resistance, mean arterial pressure, or both. Overt α-adrenoceptor effects, such as systemic vasoconstriction, are not obvious unless large doses are used.Clinical Use
Epinephrine is used in a variety of clinical situations, and although concern has been expressed about the use of epinephrine in asthma, it is still used extensively for the management of acute attacks.Clinical Use
The ability of epinephrine to stimulate β2-receptors hasled to its use by injection and by inhalation to relaxbronchial smooth muscle in asthma and in anaphylacticreactions. Several OTC preparations (e.g., Primatene, Bronkaid)used for treating bronchial asthma use E. It is also usedin inhibiting uterine contraction. Because of its α-activity, Eis used to treat hypotensive crises and nasal congestion, toenhance the activity of local anesthetics, and as a constrictorin hemorrhage.In addition, E is used in the treatment of open-angle glaucoma,where it apparently reduces intraocular pressure byincreasing the rate of outflow of aqueous humor from theanterior chamber of the eye. The irritation often experiencedon instillation of E into the eye has led to the developmentof other preparations of the drug that potentially are not asirritating. One such example is dipivefrin.
Side effects
Patients treated with recommended dosages of epinephrine will complain of feeling nervous or anxious. Some will have tremor of the hand or upper extremity and many will complain of palpitations. Epinephrine is dangerous if recommended dosages are exceeded or if the drug is used in patients with coronary artery disease, arrhythmias, or hypertension. The inappropriate use of epinephrine has resulted in extreme hypertension and cerebrovascular accidents, pulmonary edema, angina, and ventricular arrhythmias, including ventricular fibrillation. At recommended dosages, adverse effects from inhaled isoproterenol are infrequent and not serious. When excessive dosages are used, tachycardia, dizziness, and nervousness may occur, and some patients may have arrhythmias.Safety Profile
Human poison by subcutaneous route. Experimental poison by ingestion, skin contact, subcutaneous, intraperitoneal, intravenous, and intramuscular routes. Human systemic effects: cardiomyopathy includmg infarction, arrhythmias. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. Used as an adrenergic, sympathomimetic, vasoconstrictor, bronchodilator, and cardiac stimulant.Synthesis
Epinephrine, L-1-(3,4-dihydroxyphenyl)-2-methylaminoethanol (11.1.2), is obtained from the adrenal glands tissue of livestock [1,2] as well as in a synthetic manner. Epinephrine is synthesized from ω-chloro-3,4-dihydroxyacetophenone—chloroacetylpyrocatechine—the reaction of which with excess of methylamine gives ω-methylamino-3,4- dihydroxyacetophenone (11.1.1). Reduction of this using hydrogen over Raney nickel, or action of aluminum amalgam, or electrolytic reduction gives D,L-epinephrine (11.1.2) [3–9], which is separated into isomers using (+) tartaric acid .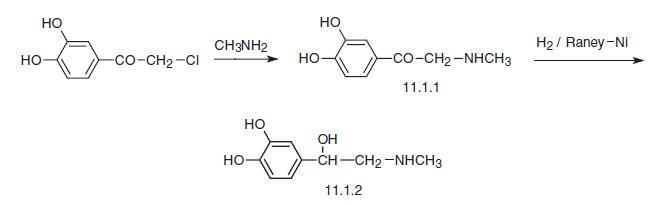
Veterinary Drugs and Treatments
Epinephrine is employed primarily in veterinary medicine as a treatment for anaphylaxis or cardiac resuscitation. Because of its vasoconstrictive properties, epinephrine is added to local anesthetics to retard systemic absorption and prolong effect.Drug interactions
Potentially hazardous interactions with other drugsAlpha-blockers: avoid with tolazoline.
Anaesthetics: increased risk of arrhythmias if given with volatile anaesthetics.
Antidepressants: increased risk of arrhythmias and hypertension if given with tricyclics; MAOIs and moclobemide may cause hypertensive crisis.
Beta-blockers: increased risk of severe hypertension and bradycardia.
Clonidine: possible increased risk of hypertension.
Dopaminergics: effects possibly increased by entacapone; avoid concomitant use with rasagiline.
Guanethidine: increased risk of hypertension.
Sympathomimetics: effects possibly enhanced by dopexamine.
Metabolism
Epinephrine is ultimately metabolized by COMT and MAO to 3-methoxy-4-hydroxy-mandelic acid (vanillylmandelic acid), which is excreted as the sulfate or glucuronide in the urine. Only a very small amount is excreted unchanged.Purification Methods
L-Adrenaline has been recrystallised from EtOH/AcOH/NH3 [Jensen J Am Chem Soc 57 1765 1935]. Itis sparingly soluble in H2O, readily in acidic or basic solutions but insoluble in aqueous NH3, alkali carbonate solutions, EtOH, CHCl3, Et2O or Me2CO. It has also been purified by dissolving in dilute aqueous acid, then precipitating it by adding dilute aqueous ammonia or alkali carbonates. It is readily oxidised in air and turns brown on exposure to light and air. (Epinephrine readily oxidises in neutral alkaline solution. This can be diminished if a little sulfite is added). Store it in the dark under N2. [Lewis Br J Pharmacol Chemother 9 488 1954]. The hydrogen oxalate salt has m 191-192o(dec, evacuated capillary) after recrystallisation from H2O or EtOH [Pickholz J Chem Soc 928 1945]. [Beilstein 13 H 830, 13 III/IV 2927.]Toxicity evaluation
Epinephrine is available in nebulized racemic dosage form for inhalation.Intoxication from catecholamine usually results from iatrogenic overdoses, accidental intravenous administration, and the injection of solution intended for nebulization. High concentrations of dopamine present inside of a cell than there are vesicles to store it in, oxidative stress can occur and cause damage or death to the cell. It is thought that dopamine overload causes biochemical damage to cellular mitochondria, that provide the cell with all of the energy it requires to function, resulting in death of the cell. Catecholamines produced circulatory changes that reversed propofol anesthesia in animal models.Preparation Products And Raw materials
Epinephrine Supplier
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +8617531153977 | allison@yan-xi.com | China | 5856 | 58 | |
| +86-0533-2185556 +8617865335152 |
Mandy@hangyubiotech.com | China | 10986 | 58 | |
| +86-371-66670886 | info@dakenam.com | China | 18940 | 58 | |
| +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21634 | 55 | |
| +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 | |
| +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1001 | 58 | |
| +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29879 | 58 | |
| +86-755-89396905 +86-15013857715 |
admin@nexconn.com | China | 10311 | 58 | |
| 18631714998 | sales@czwytech.com | CHINA | 904 | 58 | |
| +86-86-5926051114 +8618959220845 |
sales@amoychem.com | China | 6383 | 58 |
Related articles
The Pharmacodynamics of L(-)-Epinephrine and Its Role in Anaphylaxis Management
L(-)-Epinephrine is a sympathomimetic drug that activates beta-1 and beta-2 receptors, providing relief from asthma, croup, anaphylaxis, and allergic reactions, with rapid metabolism and elimination.
Dec 26,2023
L(-)-Epinephrine: Physiological effects and clinical applications
L(-)-Epinephrine regulates blood pressure, heart rate, metabolism, and the immune system. It is used in resuscitation and anesthesia.
Aug 2,2023
Epinephrine stimulates both the alpha- and beta-adrenergic systems, causes systemic vasoconstriction and gastrointestinal relaxation, stimulates the heart, and dilates bronchi and cerebral vessels. It is also used as a vasoconstrictor, car
Dec 22,2021
Epinephrine Spectrum
51-43-4, EpinephrineRelated Search
The What'sApp is temporarily not supported in mainland China