Chemical Properties
Riboflavin is moderately soluble in water (10–13 mg/dl) and ethanol but insoluble in ether, chloroform, and acetone. It is soluble but unstable under alkaline conditions.
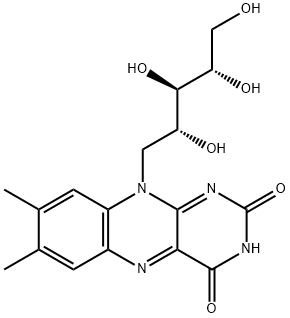
The catalytic functions of riboflavin are carried out primarily at positions N-1, N-5, and C-4 of the isoalloxazine nucleus. In addition, the methyl group at C-8 participates in covalent bonding with enzyme proteins.

Uses
People most commonly use riboflavin to prevent riboflavin deficiency, for migraine, and for high levels of homocysteine in the blood. It's also used for acne, muscle cramps, and many other conditions, but there is no good scientific evidence to support these other uses.
Side effects
In some people, riboflavin can cause the urine to turn a bright yellow color. It may also cause nausea.
Toxicology
Poison by intravenous route. Moderately toxic by intraperitoneal and subcutaneous routes. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Source
Riboflavin is widely found in both plant- and animal-based foods, including milk, meat, eggs, nuts, enriched flour, and green vegetables.
Dosage
When taken by mouth: Riboflavin is likely safe for most people in doses of up to 400 mg daily. In some people, riboflavin can cause the urine to turn a bright yellow color. It may also cause nausea. Pregnancy and breast-feeding: Riboflavin is likely safe when consumed in amounts commonly found in foods. The recommended intake is 1.4 mg daily during pregnancy and 1.6 mg daily during lactation.
Children: Riboflavin is likely safe for most children when consumed in amounts commonly found in foods. Higher doses of 100-200 mg daily have also been safely used.