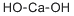
Calciumdihydroxid
Bezeichnung:Calciumdihydroxid
CAS-Nr1305-62-0
Englisch Name:Calcium hydroxide
CBNumberCB9853016
SummenformelCaH2O2
Molgewicht74.09
MOL-Datei1305-62-0.mol
Synonyma
Calciumdihydroxid
Marmorkalkhydrat
Gel?schter Kalk
Calciumdihydroxid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 580 °C |
| Siedepunkt | 2850 °C |
| chüttdichte | 400kg/m3 |
| Dichte | 2.24 g/mL at 25 °C (lit.) |
| Brechungsindex | 1.574 |
| storage temp. | Store at +5°C to +30°C. |
| Löslichkeit | 1.7g/l |
| Aggregatzustand | Solid |
| pka | 12.6[at 20 ℃] |
| Farbe | White |
| Geruch (Odor) | Odorless |
| Säure-Base-Indikators(pH-Indikatoren) | 12.4 |
| PH | 11.27(1 mM solution);12.2(10 mM solution);12.46(100 mM solution); |
| Wasserlöslichkeit | 1.65 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| Merck | 14,1673 |
| Solubility Product Constant (Ksp) | pKsp: 5.26 |
| Expositionsgrenzwerte | ACGIH: TWA 5 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 5 mg/m3 |
| Kennzeichnung gefährlicher | Xi,C |
| R-Sätze: | 41-34-37/38 |
| S-Sätze: | 26-39-45-36/37/39-27 |
| RIDADR | 3262 |
| OEB | B |
| OEL | TWA: 5 mg/m3 |
| WGK Germany | 1 |
| RTECS-Nr. | EW2800000 |
| F | 34 |
| TSCA | Yes |
| HS Code | 2825 90 19 |
| HazardClass | 8 |
| PackingGroup | III |
| Giftige Stoffe Daten | 1305-62-0(Hazardous Substances Data) |
| Toxizität | LD50 orally in rats: 7.34 g/kg (Smyth) |


