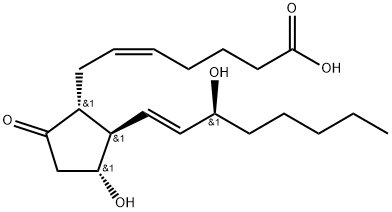
Dinoproston
Bezeichnung:Dinoproston
CAS-Nr363-24-6
Englisch Name:Prostaglandin E2
CBNumberCB8461799
SummenformelC20H32O5
Molgewicht352.47
MOL-Datei363-24-6.mol
Synonyma
Dinoproston
Dinoproston physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 66-68 °C |
| alpha | -85.5 º (c=1, C2H5OH) |
| Siedepunkt | 406.07°C (rough estimate) |
| Dichte | 1.0601 (rough estimate) |
| Brechungsindex | 1.6120 (estimate) |
| storage temp. | -20°C |
| Löslichkeit | ethanol: 1 mg/mL |
| Aggregatzustand | powder |
| pka | pKa 4.77± 0.09(H2O,t=25±0.1,I=0.1(NaCl)) (Uncertain) |
| Farbe | Clear yellow to amber |
| Biologische Quelle | synthetic (organic) |
| Wasserlöslichkeit | insoluble |
| Merck | 14,7877 |
| BRN | 4709356 |
| Stabilität | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| InChIKey | XEYBRNLFEZDVAW-ARSRFYASSA-N |
| CAS Datenbank | 363-24-6(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | T |
| R-Sätze: | 60-22-61 |
| S-Sätze: | 53-22-26-36/37/39-45 |
| WGK Germany | 3 |
| RTECS-Nr. | UK8000000 |
| F | 8-10 |
| HS Code | 29375000 |


