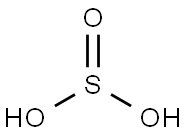
Schwefligesure
Bezeichnung:Schwefligesure
CAS-Nr7782-99-2
Englisch Name:Sulfurous Acid
CBNumberCB6160852
SummenformelH2O3S
Molgewicht82.08
MOL-Datei7782-99-2.mol
Synonyma
Schwefligesure
Schwefligesure physikalisch-chemischer Eigenschaften
| Dichte | 1.03 g/mL at 25 °C(lit.) |
| Dampfdichte | 2.3 (vs air) |
| Löslichkeit | solution of SO2 in H2O |
| pka | 1.92(at 25℃) |
| Aggregatzustand | Liquid |
| Farbe | Colorless |
| PH | pKa1= 1.658, pKa2 = 6.820(25℃) |
| Wasserlöslichkeit | Miscible with water. |
| Sensitive | Air Sensitive |
| Merck | 14,8976 |
| Dielectric constant | 15.6(0℃) |
| Stabilität | Stable. Incompatible with strong bases. |
| InChIKey | LSNNMFCWUKXFEE-UHFFFAOYSA-N |
| LogP | -1.579 (est) |
| CAS Datenbank | 7782-99-2(CAS DataBase Reference) |
| EPA chemische Informationen | Sulfurous acid (7782-99-2) |
| Kennzeichnung gefährlicher | C,Xn |
| R-Sätze: | 20-34-36/38 |
| S-Sätze: | 26-36/37/39-45 |
| RIDADR | UN 1833 8/PG 2 |
| WGK Germany | 1 |
| RTECS-Nr. | WT2775000 |
| F | 10-23 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 28111990 |


