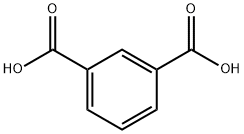
Isophthalsure
Bezeichnung:Isophthalsure
CAS-Nr121-91-5
Englisch Name:Isophthalic acid
CBNumberCB3262667
SummenformelC8H6O4
Molgewicht166.13
MOL-Datei121-91-5.mol
Synonyma
Isophthalsure
1,3-Benzoldicarbons?ure
m-Phthals?ure
Isophthalsure physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 341-343 °C (lit.) |
| Siedepunkt | 214.32°C (rough estimate) |
| Dichte | 1,54 g/cm3 |
| Dampfdruck | 0Pa at 25℃ |
| Brechungsindex | 1.5100 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| Löslichkeit | 0.12g/l |
| Aggregatzustand | Crystalline Powder |
| pka | 3.54(at 25℃) |
| Farbe | White to off-white |
| PH | 3.33(1 mM solution);2.76(10 mM solution);2.24(100 mM solution) |
| Wasserlöslichkeit | 0.01 g/100 mL (25 ºC) |
| Merck | 14,5197 |
| BRN | 1909332 |
| Dielectric constant | 1.4(Ambient) |
| Stabilität | Stable. Incompatible with strong oxidizing agents, strong bases. |
| InChIKey | QQVIHTHCMHWDBS-UHFFFAOYSA-N |
| LogP | 1.66 at 25℃ |
| Kennzeichnung gefährlicher | Xi |
| R-Sätze: | 36/37/38 |
| S-Sätze: | 24/25-36-26 |
| WGK Germany | 2 |
| RTECS-Nr. | NT2007000 |
| Selbstentzündungstemperatur | 1198 °F |
| TSCA | Yes |
| HS Code | 29173980 |
| Giftige Stoffe Daten | 121-91-5(Hazardous Substances Data) |


