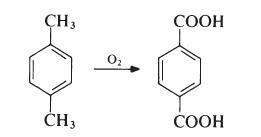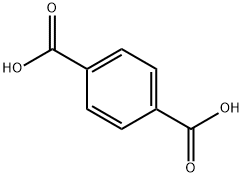
Terephthalsure
Bezeichnung:Terephthalsure
CAS-Nr100-21-0
Englisch Name:Terephthalic acid
CBNumberCB8852557
SummenformelC8H6O4
Molgewicht166.13
MOL-Datei100-21-0.mol
Synonyma
Terephthalsure
p-Phthals?ure
1,4-Benzoldicarbons?ure
Terephthalsure physikalisch-chemischer Eigenschaften
| Schmelzpunkt | >300 °C (lit.) |
| Siedepunkt | 214.32°C (rough estimate) |
| Dichte | 1.58 g/cm3 at 25 °C |
| Dampfdruck | <0.01 mm Hg ( 20 °C) |
| Brechungsindex | 1.5100 (estimate) |
| Flammpunkt | 260°C |
| storage temp. | Sealed in dry,Room Temperature |
| Löslichkeit | 15mg/l (experimental) |
| Aggregatzustand | Crystalline Powder |
| pka | 3.51(at 25℃) |
| Farbe | White |
| PH | 3.36(1 mM solution);2.79(10 mM solution);2.26(100 mM solution) |
| Wasserlöslichkeit | slightly soluble in water (0,017 g/L at 25°C) |
| Merck | 14,9162 |
| BRN | 1909333 |
| Expositionsgrenzwerte | ACGIH: TWA 10 mg/m3 |
| Dielectric constant | 1.5(Ambient) |
| Stabilität | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Kennzeichnung gefährlicher | Xi |
| R-Sätze: | 36/37/38 |
| S-Sätze: | 26-36 |
| WGK Germany | 3 |
| RTECS-Nr. | WZ0875000 |
| Selbstentzündungstemperatur | 925 °F |
| TSCA | Yes |
| HS Code | 2917 36 00 |
| Giftige Stoffe Daten | 100-21-0(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: > 6400 mg/kg |