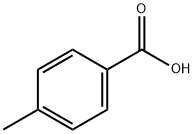
p-Toluylsäure
Bezeichnung:p-Toluylsäure
CAS-Nr99-94-5
Englisch Name:p-Toluic acid
CBNumberCB7854776
SummenformelC8H8O2
Molgewicht136.15
MOL-Datei99-94-5.mol
Synonyma
p-Toluylsure
p-Toluylsäure
p-Toluylsäure physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 177-180 °C (lit.) |
| Siedepunkt | 274-275 °C (lit.) |
| Dichte | 1,06 g/cm3 |
| chüttdichte | 700kg/m3 |
| Dampfdruck | 0.02 hPa (70 °C) |
| Brechungsindex | 1.5120 (estimate) |
| Flammpunkt | 181°C |
| storage temp. | Store below +30°C. |
| Löslichkeit | 0.3g/l |
| pka | 4.36(at 25℃) |
| Aggregatzustand | Powder |
| Farbe | White to slightly yellow-cream |
| PH | 3.73(1 mM solution);3.2(10 mM solution);2.69(100 mM solution) |
| Wasserlöslichkeit | <0.1 g/100 mL at 19 ºC |
| Merck | 14,9535 |
| BRN | 507600 |
| Stabilität | Stable. Incompatible with strong oxidizing agents, strong bases. |
| InChIKey | LPNBBFKOUUSUDB-UHFFFAOYSA-N |
| Kennzeichnung gefährlicher | Xn |
| R-Sätze: | 22 |
| S-Sätze: | 22-24/25 |
| WGK Germany | 1 |
| RTECS-Nr. | XU1575000 |
| Selbstentzündungstemperatur | 570 °C |
| TSCA | Yes |
| HS Code | 29163900 |
| Toxizität | LD50 orally in Rabbit: 400 mg/kg |

