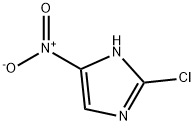
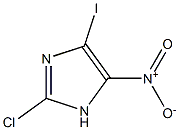
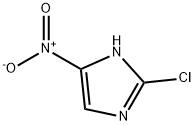
Example 9 Preparation of 2-chloro-4-nitroimidazole: 2-chloro-5-iodo-4-nitroimidazole (273 mg, 1.0 mmol) was dissolved in anhydrous ethanol (5 mL), followed by sequential addition of triethylamine (420 μL, 3.0 mmol) and 10% palladium-carbon catalyst (27 mg) to the solution. The reaction mixture was hydrogenated by stirring for 3 hours at room temperature and under an atmosphere of hydrogen at atmospheric pressure. Upon completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated under reduced pressure to afford the colorless solid product 2-chloro-4-nitroimidazole (124 mg, 84.1% yield). The product was characterized by 1H-NMR (DMSO-d6): δ 8.44 (1H, s), 14.19 (1H, bs).
Example 11 Preparation of 2-chloro-4-nitroimidazole: 2-chloro-5-iodo-4-nitroimidazole (545 mg, 2.0 mmol) was dissolved in anhydrous ethanol (10 mL), followed by sequential addition of triethylamine (840 μL, 6.0 mmol) and 10% palladium-carbon catalyst (54 mg) to the solution. The reaction mixture was hydrogenated under hydrogen pressure of 4 kg/cm2 in a pearl reduction apparatus. Upon completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated under reduced pressure to give the colorless solid product 2-chloro-4-nitroimidazole (246 mg, 83.4% yield). The product was characterized by 1H-NMR (DMSO-d6): δ 8.44 (1H, s), 14.19 (1H, bs).