Application of 1,1,3,3-Tetramethyldisiloxane
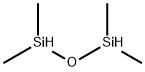
1,1,3,3-Tetramethyldisiloxane (Tetramethyldisiloxane; (CH3)2HSiOSiH(CH3)2; 1,3-Dihydrotetramethyldisiloxane; Bis(dimethylsilyl)-oxide; Bis(dimethylsilyl)oxide; TMDSO) is soluble in many organic solvents, such as aromatic hydrocarbon and petroleum hydrocarbons, and so on[1] .

Meanwhile, 1,1,3,3-Tetramethyldisiloxane (TMDS) is a kind of widely used organic silicon intermediates and usually be used as organic silicon blocking agent in the synthesis[2]. Due to containing reactive Si-H groups in the molecular structure, it can be used in the synthesis of copolymer macromolecule by hydrosilylation. The synthesis of copolymer macromolecule can be made into a series of reactive silicone oil. In organic synthesis, it finds an application as an effective reducing agent in platinum-catalyzed reduction of carboxamides to amines as well as in the Au/TiO2-catalyzed hydrosilylation of carbonyl compounds (e.g., reduction of aldehydes or ketones) relative to monohydrosilanes. TMDS is a highly reactive reducing reagent in the Au/TiO2-catalyzed hydrosilylation of carbonyl compounds relative to monohydrosilanes. The reduction of aldehydes or ketones with TMDS can be performed on many occasions at ambient conditions within short reaction times and at low loading levels of gold[3].
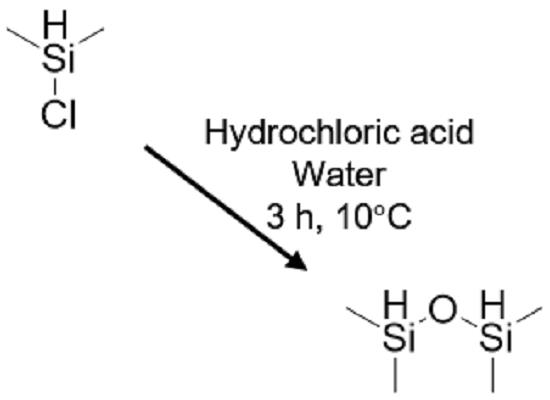
In addtion, It is used as an intermediate for preparing other organosilicon compounds. It is also used in non-aqueous polymer preparation as well as a laboratory reagent. Ioannis N.[4] et al found that gold nanoparticles supported on TiO2 (0.11% mol) catalyze at room temperature and at extremely mild conditions the unprecedented oxidative cycloaddition of 1,1,3,3-tetramethyldisiloxane to alkynes, forming substituted 2,5-dihydro-1,2,5-oxadisiloles, with concomitant evolution of hydrogen gas. For the majority of the substrates, the yields are exceptional (up to 99%). The reaction proceeds at room temperature, tolerates a variety of functional groups, and can be performed in several solvents. Ireneusz[5] et al. Found that the study of the selective mono-functionalization of 1,1,3,3-tetramethyldisiloxane (TMDSO) with vinyl-substituted silicon derivatives through the hydrosilylation reaction. An investigation of the use of platinum-and rhodium-based catalysts in the reactions between TMDSO and exemplarvinyl-containing silicon derivatives has enabled us to identify the most efficient catalyst, the application of which led to the selective partial functionalization of the disiloxane reagent and the simultaneous formation of only β regioisomers.
References
[1] https://www.chemicalbook.com/ProductChemicalPropertiesCB5355451_EN.htm
[2] http://www.organic-chemistry.org/chemicals/reductions/tetramethyldisiloxane-TMDSO.shtm
[3] Vasilikogiannaki E, Titilas I, Gryparis C, et al. Efficient hydrosilylation of carbonyl compounds by 1, 1, 3, 3-tetramethyldisiloxane catalyzed by Au/TiO2[J]. Tetrahedron, 2014, 70(36): 6106-6113.
[4] Lykakis I N, Psyllaki A, Stratakis M. Oxidative cycloaddition of 1, 1, 3,3-tetramethyldisiloxane to alkynes catalyzed by supported gold nanoparticles[J]. Journal of the American Chemical Society, 2011, 133(27): 10426-10429.
[5]Januszewski R, Kownacki I, Maciejewski H, et al. An Efficient Catalytic Route for the Synthesis of Silane Coupling Agents Based on the 1, 1, 3, 3-Tetramethyldisiloxane Core[J]. European Journal of Inorganic Chemistry, 2017, 2017(4): 851-856.
You may like
See also
Lastest Price from 1,1,3,3-Tetramethyldisiloxane manufacturers

US $0.00/kg2025-06-12
- CAS:
- 3277-26-7
- Min. Order:
- 1kg
- Purity:
- 99% Min.
- Supply Ability:
- 20mt per month

US $0.00/KG2025-04-21
- CAS:
- 3277-26-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt