Application research of D-2-phenylglycine
Introduction
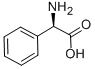
Optically active D-amino acids are widely used in the pharmaceutical industry as intermediates for the synthesis of semisynthetic antibiotics and pesticides. Among them, the demand for D-2-phenylglycine (Figure 1) for the synthesis of drugs such as aspoxicillin, cefbuperazone, and cefpiramide has been increasing. The efficiency of various procedures for the production of D-2-phenylglycine have been studied, such as the production from its keto-analogue by D-amino acid aminotransferase and from D,L-2-phenylglycine using isoelectrically trapped penicillin G acylase.The D-amino acid aminotransferase system has a low reaction rate and D-2- phenylglycine is transformed from 2-oxo-2-phenylacetic acid with a molar yield of 2%. The conversion system using penicillin G acylase requires a multicompartment electrolyzer with an isoelectric membrane to prevent the reverse process,the hydrolysis of 4-hydroxyphenylacetyl-L-phenylglycine.[1]

Enantioselective N-acetylation of D-2-phenylglycine
The demand for D-2-phenylglycine used to synthesize semisynthetic antibiotics and pesticides is increasing. Several studies have reported the production of D-2-phenylglycine using bacterial enzymes. AlthoughL-2-phenylglycine can easily be degraded and removed from cultures containing the racemic 2-phenylglycine mixture by growing cells, the two isomers are metabolized via phenyglyoxylate and benzoate by the sameroute. Although the nitrile hydratase of immobilized cells of Pseudomonas aeruginosa 10145 in 25 ml batch cultures can efficient lyconvert 2-phenyl-2-aminoacetonitrile to D-2-phenylglycine with an enantiomer excess of 98 %, the production system has not yet been up-scaled. Along these lines, the N-acetylation reported here probably would improve the solubility of 2-phenylglycine, making it easier to separate the remaining D-2-phenylglycine and the produced (2S)-2-acetylamino-2-phenylacetic acid based on their differing solubilities.[1]
Takenaka et al. have isolated a Chryseobacterium sp. that selectively transformed the L-form of racemic D,L-2-phenylglycine to (2S)-2-acetylamide-2-phenylacetic acid with a molar yield of 50% and an enantiomer excess of >99.5% under optimal culture conditions, consequently resulting in 99% pure D-2-phenylglycine remaining in the culture. The enantioselective N-acetylation was catalyzed by an acetyl-CoA-dependent N-acetyltransferase whose synthesis was induced by L-2-phenylglycine. The enzyme differed from previously reported bacterial arylamine N-acetyltransferases in molecular mass and substrate specificity. The relative activity ratio of the enzyme with the substrates L-2-phenylglycine, D-2-phenylglycine, 2-(2-chlorophenyl)glycine, and 5-aminosalicylic acid (a good substrate of arylamine N-acetyltransferase) was 100:0:56.9:5.49, respectively. The biotransformation by the N-acetyltransferase-producing bacterium reported here could constitute a new preparative route for the enzymatic resolution of D,L-2-phenylglycine.[1]
Amino acids functionalized magnetite nanoparticles production
Rodriguez et al. report the synthesis of magnetite nanoparticles (MNP) and their functionalization with glycine (MNPGly), β-alanine (MNPAla), L-phenylalanine (MNPPhAla), D-2-phenylglycine (MNPPhGly) amino acids. The functionalized nanoparticles were characterized by Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffraction (XRD), electron paramagnetic resonance (EPR), vibrating sample magnetometry (VSM), Mössbauer spectroscopy (MS), magnetic hyperthermia (MH), dynamic light scattering and zeta potential. The functionalized nanoparticles had isoelectric points (IEP) at pH 4.4, 5.8, 5.9 and 6.8 for samples MNPGly, MNPAla, D-2-phenylglycine(MNPPhGly) and MNPPhAla, respectively, while pure magnetite had an IEP at pH 5.6. In the MH experiments, the samples showed specific absorption rate (SAR) of 64, 71, 74, 81 and 66 W/g for MNP, MNPGly, MNPAla, D-2-phenylglycine, and MNPPhAla, respectively. Rodriguez et al. used a flow cytometric technique to determine the cellular magnetic nanoparticles plus amino acids content. Magnetic fractionation and characterization of Resovist® magnetic nanoparticles were performed for applications in magnetic particle imaging (MPI). Rodriguez et al. have also studied the antiproliferative and antiparasitic effects of functionalized MNPs. Overall, the data showed that the functionalized nanoparticles have great potential for using as environmental, antitumor, antiparasitic agents and clinical applications.[2]
Hantzsch esters synthesis
In this work, D-2-phenylglycine (APG)-functionalized magnetic nanocatalyst (Fe3O4@SiO2@PTS-APG) was designed and successfully prepared in order to implement the principles of green chemistry for the synthesis of polyhydroquinoline (PHQ) and 1,4-dihydropyridine (1,4-DHP) derivatives under ultrasonic irradiation in EtOH. After preparing of the nanocatalyst, its structure was confirmed by different spectroscopic methods or techniques including Fourier transform infrared (FTIR) spectroscopy, energy-dispersive X-ray spectroscopy (EDS), field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), vibrating sample magnetometer (VSM) and thermal gravimetric analysis (TGA). The performance of Fe3O4@SiO2@PTS-APG nanomaterial, as a heterogeneous catalyst for the Hantzsch condensation, was examined under ultrasonic irradiation and various conditions. The yield of products was controlled under various conditions to reach more than 84% in just 10 min, which indicates the high performance of the nanocatalyst along with the synergistic effect of ultrasonic irradiation. The structure of the products was identified by melting point as well as FTIR and 1H-NMR spectroscopic methods. The Fe3O4@SiO2@PTS-APG nanocatalyst is easily prepared from commercially available, lower toxic and thermally stable precursors through a cost-effective, highly efficient and environmentally friendly procedure. The advantages of this method include simplicity of the operation, reaction under mild conditions, the use of an environmentally benign irradiation source, obtaining pure products with high efficiency in short reaction times without using a tedious path, which all of them address important green chemistry principles. Finally, a reasonable mechanism is proposed for the preparation of polyhydroquinoline (PHQ) and 1,4-dihydropyridine (1,4-DHP) derivatives in the presence of Fe3O4@SiO2@PTS-APG bifunctional magnetic nanocatalyst.[3]
References
1. Takenaka S, Honma Y, Yoshida K, Yoshida K. Enantioselective N-acetylation of 2-phenylglycine by an unusual N-acetyltransferase from Chryseobacterium sp. Biotechnol Lett. 2013;35(7):1053-1059. doi:10.1007/s10529-013-1172-z
2. Rodriguez AFR, Dos Santos CC, Lüdtke-Buzug K, et al. Evaluation of antiplasmodial activity and cytotoxicity assays of amino acids functionalized magnetite nanoparticles: Hyperthermia and flow cytometry applications. Mater Sci Eng C Mater Biol Appl. 2021;125:112097. doi:10.1016/j.msec.2021.112097
3. Shakib P, Dekamin MG, Valiey E, Karami S, Dohendou M. Ultrasound-Promoted preparation and application of novel bifunctional core/shell Fe3O4@SiO2@PTS-APG as a robust catalyst in the expeditious synthesis of Hantzsch esters. Sci Rep. 2023;13(1):8016. Published 2023 May 17. doi:10.1038/s41598-023-33990-7
You may like
See also
Lastest Price from D-2-Phenylglycine manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 875-74-1
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 500kgs

US $0.00-0.00/kg2025-04-21
- CAS:
- 875-74-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt