1,1,3,3-Tetramethyldisiloxane: A Versatile Organosilane for Safe and Selective Organic Synthesis Processes
General Description
1,1,3,3-Tetramethyldisiloxane is a key bifunctional organosilane used in organic synthesis, known for stability in neutral pH but high reactivity with strong bases. 1,1,3,3-Tetramethyldisiloxane serves as a proficient reducing agent, crucial in producing higher molecular weight hydride-terminated polydimethylsiloxanes. Its weakly hydridic Si−H bond allows for selective reduction of various organic functional groups. In the reduction of phosphine oxides, 1,1,3,3-Tetramethyldisiloxane plays a vital role, offering scalability and practicality in converting waste products to valuable phosphines. Despite its flammability hazard, its safety profile and effectiveness make 1,1,3,3-Tetramethyldisiloxane an ideal silicon-based reagent for safe and selective organic synthesis processes.

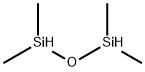
Figure 1. 1,1,3,3-Tetramethyldisiloxane
Chemical Characteristics
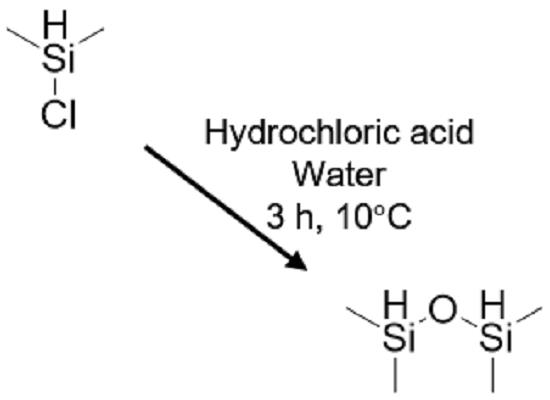
1,1,3,3-Tetramethyldisiloxane is a pivotal bifunctional organosilane within the family of hydride-terminated polydimethylsiloxanes. It is synthesized through the meticulous hydrolysis of chlorodimethylsilane. This process warrants caution due to the liberation of hydrogen chloride (HCl) and the susceptibility of the Si−H bond. Elevated temperatures and prolonged reaction times can trigger the hydrolysis of the Si−H bond, resulting in the formation of oligomeric siloxanes and dihydrogen. 1,1,3,3-Tetramethyldisiloxane exhibits stability in neutral or near-neutral pH environments but demonstrates high reactivity towards strong bases and to a lesser extent, acids. Notably, it possesses an autoignition temperature of 245 °C. 1,1,3,3-Tetramethyldisiloxane serves as a proficient reducing agent, primarily employed as an end-capper in the production of higher molecular weight hydride-terminated polydimethylsiloxanes. The Si−H bond in 1,1,3,3-Tetramethyldisiloxane is weakly hydridic, rendering it less reactive and more selective for reducing various organic functional groups. This characteristic is attributed to the relatively low electronegativity values of silicon and hydrogen. Additionally, modifications in the nonreactive groups attached to the silicon can alter the electronic and steric properties of organosilane reductants. Ionic and catalyzed organosilane reductions, often facilitated by acid catalysts, are extensively studied due to the compromised reactivity of the Si−H bond. Consequently, functional groups capable of generating stable carbocations are readily reduced under mild conditions by organosilanes. 1
Reduction of phosphine oxides
The reduction of phosphine oxides, such as 1,1,3,3-Tetramethyldisiloxane, plays a crucial role in various organic synthesis processes, particularly in the synthesis of aryl and alkylphosphines, important components in many organometallic-catalyzed reactions. Traditionally, these phosphine oxides were considered waste products, but recent developments have highlighted their potential as valuable preligands. Several methods have been explored for the reduction of phosphine oxides, including the use of hexachlorodisilane, aluminum hydrides, SmI2/HMPA, Cp2TiCl2/Mg, and Bi/TiO2. However, these methods often lack scalability and practicality for industrial applications. Recent advancements have addressed these limitations. The Lemaire group introduced a practical and scalable process utilizing 1,1,3,3-Tetramethyldisiloxane as the silane reductant and catalytic Ti(O-i-Pr)4. This method demonstrated high yields and scalability, converting phosphine oxides back to more useful phosphines effectively. Additionally, an InBr3-catalyzed 1,1,3,3-Tetramethyldisiloxane reduction system showed versatility in reducing various phosphine oxides, including phosphinic acids, with good to excellent yields. Furthermore, the Beller group demonstrated the deoxygenation of phosphine oxides using 1,1,3,3-Tetramethyldisiloxane/Cu(OTf)2, providing mild conditions and tolerance towards functional groups like ketones, esters, and olefins. This method complements the TMDS/InBr3 procedure, offering a convenient route to triarylphosphines. These advancements not only offer efficient routes to valuable phosphine compounds but also highlight the potential for scalability and commercialization, emphasizing the importance of safe and cost-effective silane reductants like 1,1,3,3-Tetramethyldisiloxane in organic synthesis processes. 2
Safety and Efficacy in Chemical Synthesis
1,1,3,3-Tetramethyldisiloxane is a comparatively safe compound widely used in organic synthesis. Notably, 1,1,3,3-Tetramethyldisiloxane does not produce monosilane (SiH4), a highly combustible and explosive gas. This is in contrast to triethoxysilane (HSi(OEt)3), which can partially convert to monosilane, posing significant safety risks including the potential for blindness due to severe eye irritation. Additionally, arylsilanes such as phenylsilane and diphenylsilane, while effective in certain reductions, have the drawback of forming pyrophoric silanes under acidic conditions. However, it's important to note that 1,1,3,3-Tetramethyldisiloxane does have a low flash point of 12°C, which means it presents a flammability hazard and necessitates careful handling procedures. Despite this, its overall physical properties make it a robust and selective reagent, suitable for various applications in organic synthesis. Considering its relative safety profile and ease of handling, 1,1,3,3-Tetramethyldisiloxane emerges as an ideal silicon-based reducing agent, particularly for larger-scale operations where safety is paramount. 2
Reference
1. Hudrlik PF. Ionic and Organometallic-Catalyzed Organosilane Reductions. Journal of the American Chemical Society. 2010; 132(28): 9929.
2. Jaan P, Gerald LL. Tetramethyldisiloxane: A Practical Organosilane Reducing Agent. Organic Process Research & Development. 2016; 20(7): 1164-1181.
You may like
Related articles And Qustion
See also
Lastest Price from 1,1,3,3-Tetramethyldisiloxane manufacturers

US $0.00/kg2025-06-12
- CAS:
- 3277-26-7
- Min. Order:
- 1kg
- Purity:
- 99% Min.
- Supply Ability:
- 20mt per month

US $0.00/KG2025-04-21
- CAS:
- 3277-26-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt