Ethyl Vanillin: Pharmacokinetic Properties and Toxicity
General Description
Ethyl vanillin, a widely used flavoring agent, demonstrates rapid absorption and urinary excretion in both animals and humans. Metabolism primarily leads to ethyl vanillic acid formation, with glucuronide and sulfate conjugates as major metabolites. While generally non-toxic at normal doses, prolonged high-dose exposure in animals can result in adverse effects such as anemia and liver changes. Ethyl vanillin also exhibits pharmacological properties and potential genotoxicity, necessitating careful monitoring and regulation in food and drug applications. Overall, understanding Ethyl vanillin's pharmacokinetic and toxicological profiles is crucial for ensuring safety and efficacy in various industries.

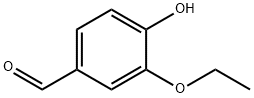
Figure 1. Ethyl Vanillin
Anti-angiogenic acivity
The chick chorioallantoic membrane (CAM), an extraembryonic membrane commonly used in vivo to measure both angiogenesis and anti-angiogenesis, was used to determine the inhibitory activity of Ethyl Vanillin(EVA) on vascular development. Retinoic acid, used as a positive control for the assay, was previously found to contain anti-angiogenic activity by down-regulating the expression and release of pro-angiogenic factors. After the 2-day treatment, retinoic acid at 1 µg/egg gave an inhibition of 75.0% in the branching patterns of blood vessels. When 0.1, 0.3, 1.0, and 3.0 µg/egg of EVA was applied onto the CAMs, the inhibition percentages in the CAM angiogenesis were 35.3%, 46.7%, 50.0%, and 65.0%, respectively. The dose required for half-maximal inhibition (IC50) of EVA was calculated to be 3.4 µg/egg. Collectively, Ethyl Vanillin possesses significant antiangiogenic activity in a dose-dependent manner.1
Anti-inflammatory activity
Ethyl Vanillin(EVA) at the oral doses of 10, 30 and 100 mg/kg exhibited an inhibition of 76.0%, 79.7% and 82.0% in vascular permeability model, respectively. In the carrageenan-induced inflammation in the air pouch as an excellent acute inflammatory model, fluid extravasation, leukocyte migration and biochemical parameters in the exudate can be easily detected. The injection of carrageenan into a subcutaneous air pouch on the dorsal surface of rats initiated an inflammatory process. In the carrageenan-induced inflammation in the air pouch, dexamethasone (DEXA, 0.01 mg/pouch) reduced the volumes of the exudates by 41.8%. EVA at 0.03, 0.1 and 0.3 mg/pouch showed an inhibition of 29.2%, 34.9% and 38.4%, respectively, with respect to the exudate volume in the control group. Total numbers of the polymorphonuclear leukocytes in the air pouches were also diminished by EVA at 0.03, 0.1 and 0.3 mg/pouch, the inhibitory percentages of which were 3.8%, 12.8% and 19.6%, respectively. In brief, Ethyl Vanillin contains acute anti-inflammatory activity.1
Toxicity
Ethyl vanillin, a compound known for its vanilla-like fragrance, is widely utilized in various industries, including food, beverages, cosmetics, and drugs, both as a flavor enhancer and a fragrance component. Despite its widespread use, understanding its toxicity profile is crucial for ensuring consumer safety. Research on human exposure to ethyl vanillin indicates minimal impact on the activity of five human CYP450 enzymes within a certain concentration range. However, direct contact with a 2% concentration of ethyl vanillin may cause mild skin irritation in humans after 48 hours. Animal studies have provided further insights into the toxicity of ethyl vanillin. While it is generally considered non-toxic at typical doses, prolonged exposure to high doses can lead to adverse effects. For instance, in rabbits given ethyl vanillin orally, anemia, diarrhea, and lack of weight gain were observed at a dose of 49 mg/kg bw/day. Similarly, in rats fed ethyl vanillin for 13 weeks at increasing dose levels, significant changes in clinical biochemistry and histological examination of the liver were noted, including hepatic peribiliary inflammatory changes and bile duct hyperplasia. However, long-term dietary exposure studies in rats over two years did not reveal any adverse effects on growth, organ weights, hematology, or histology. Nevertheless, ethyl vanillin has shown potential genotoxicity in vitro, enhancing the ability of certain substances to induce genetic changes. Additionally, ethyl vanillin exhibits various pharmacological properties, including anti-angiogenic, anti-inflammatory, and anti-nociceptive effects, attributed to its modulation of nitric oxide production and reactive oxygen species levels. Furthermore, ethyl vanillin's interaction with drugs metabolized by specific enzymes suggests the importance of regulating its use in foods and drugs to avoid adverse interactions. Notably, supplementation of powdered infant formula with ethyl vanillin has been shown to enhance safety by reducing the thermal tolerance of harmful bacteria like Cronobacter sakazakii during rehydration. In conclusion, while ethyl vanillin is generally considered safe at typical levels of exposure, careful monitoring and regulation are necessary to mitigate potential risks associated with prolonged or high-dose exposure. 2
References:
[1] HYUN-JOO JUNG. Assessment of the anti-angiogenic, anti-inflammatory and antinociceptive properties of ethyl vanillin[J]. Archives of Pharmacal Research, 2010, 33 2. DOI:10.1007/s12272-010-0217-2.Related articles And Qustion
Lastest Price from Ethyl vanillin manufacturers

US $0.00-0.00/kg2025-09-18
- CAS:
- 121-32-4
- Min. Order:
- 1kg
- Purity:
- 99.8%
- Supply Ability:
- 1000kg

US $10.00/ASSAYS2025-08-29
- CAS:
- 121-32-4
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton