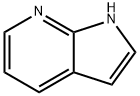
Application research of 7-Azaindole
Introduction
7-Azaindole is well-known for its ability to form bidentate hydrogen bonds with the hinge region of protein kinases, as a result, it has been identified as a privileged scaffold for kinase inhibition. A previous survey revealed that over 90 protein kinases are sensitive to 7-azaindole-based compounds, and this suggests that 7-azaindole is a useful starting point for the development of various protein kinase inhibitors. The B-Raf kinase inhibitor vemurafenib was the first FDA-approved 7-azaindole-based protein kinase inhibitor. Notably, several 7-azaindole-based protein kinase inhibitors are currently undergoing clinical evaluation, and most of them have been developed to target kinases such as colony stimulating factor receptor 1, rho-associated,coiled-coil-containing protein kinase 1 (ROCK1), aurora kinases and Janus kinase 3 (JAK3).[1]
7-Azaindole’structural features
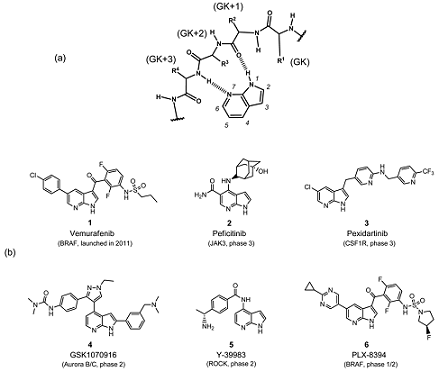
7-Azaindole is well known as a kinase privileged fragment, and has been incorporated into many kinase inhibitors as a hinge-binding element. As shown in Fig. 1a, the pyridine N atom and the pyrrole NH in the 7-azaindole ring serve as a hydrogen bond acceptor and donor, respectively, to make bidentate hydrogen bonds with the hinge region of the kinase. In addition, 7-azaindole has five modification sites where various substituents can be readily attached. Therefore recent years have seen growing interest in this 7-azaindole scaffold; more than 100000 chemical structures having 7-azaindole framework have been registered in the CAS chemical database,which is steadily increasing by the synthetic innovation and enrichment of commercially available derivatives.

Application of 7-azaindole
Figure1b shows representative kinase inhibitors having a number of substituents at various positions in the 7-azaindole ring. Among them, vemurafenib (1), a B-RAF kinase (serine–threonine kinase [STK]) inhibitor, is the first FDA-approved 7-azaindole-based kinase drug for the treatment of melanoma. Vemurafenib was discovered through lead optimization starting from a small 7-azaindole fragment, and is recognized as the first successful example of “fragment-based” drug discovery approach. Currently, several 7-azaindole-basedkinase inhibitors (2–6) are under clinical evaluation as shown in Fig. 1b. Apart from PLX-8394 (6), a second-generation BRAF kinase inhibitor, other compounds have been developed targeting various kinds of kinases including Januskinase 3 (JAK3; a cytoplasmic tyrosine kinase [TK]), colony stimulating factor 1 receptor (CSF1R; a receptor TK),aurora kinases (STK), and rho-associated, coiled-coil-containing protein kinase 1 (ROCK1; STK).15) Comprehensive survey of a drug discovery database (substructure-search using Thomson Reuters IntegritySM database18) as of March 2017) revealed that more than 90 kinds of kinases have been shown sensitive to 7-azaindole-based kinase inhibitors—sufficient to cover the whole human kinome.These data suggest that 7-azaindole fragment can be useful as a starting point of medicinal chemistry targeting various kinases.[2]
In the current portfolio, there is a lot of interest in the 7-azaindole building block for drug discovery. The creation of synthetic, sophisticated methods for the modification of 7-azaindoles is a promising area of research. This review covers the structure–activity relationship of 7-azaindole analogs, which have been shown to be effective anticancer agents in the literature of the past two decades. Positions 1, 3 and 5 of the 7-azaindole ring are the most active sites. Disubstitution is used for the synthesis of a new analog of the7-azaindole moiety. All positions are used to create novel molecules that are effective anticancer agents.The alkyl, aryl carboxamide group and heterocyclic ring are the most successful types of substitution.[3]
7-Azaindole-coumaranone hybrids and their analogues
In this study, Qhobosheane et al. determined the inhibition profiles of novel mono- and disubstituted derivatives of 7-azaindole-coumaranone hybrids on various disease-related protein kinases. Eight hit compounds were identified, including a potent Haspin inhibitor with an IC50 value of 0.15μM. An interesting observation was that all active monosubstituted compounds displayed dual inhibition for Haspin and GSK-3β, while disubstituted derivatives inhibited GSK-3β and LmCK1 from Leishmania major parasite. Analyses of structure activity relationships (SARs) also revealed that mono-substitution with para-fluorobenzyloxy ring produced an equipotent inhibition of Haspin and GSK-3β. Haspin and GSK-3β are relevant targets for developing new anticancer agents while LmCK1 is an innovative target for leishmanicidal drugs. Novel compounds reported in this paper constitute promising starting points for the development of new anticancer and leishmanicidal drugs.[1]
References
1.Qhobosheane MA, Beteck RM, Baratte B, et al. Exploration of 7-azaindole-coumaranone hybrids and their analogues as protein kinase inhibitors. Chem Biol Interact. 2021;343:109478. doi:10.1016/j.cbi.2021.109478
2.Irie T, Sawa M. 7-Azaindole: A Versatile Scaffold for Developing Kinase Inhibitors. Chem Pharm Bull (Tokyo). 2018;66(1):29-36. doi:10.1248/cpb.c17-00380
3.Sharma N, Chaudhary A, Sachdeva M. An insight into the structure-activity relationship studies of anticancer medicinal attributes of 7-azaindole derivatives: a review. Future Med Chem. 2023;15(24):2309-2323. doi:10.4155/fmc-2023-0216
You may like
Related articles And Qustion
See also
Lastest Price from 7-Azaindole manufacturers

US $9.85/KG2025-04-21
- CAS:
- 271-63-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 m
US $0.00-0.00/KG2025-04-04
- CAS:
- 271-63-6
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1Ton