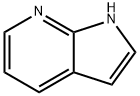
7-Azaindole: Uses and Synthesis
What is 7-Azaindole?
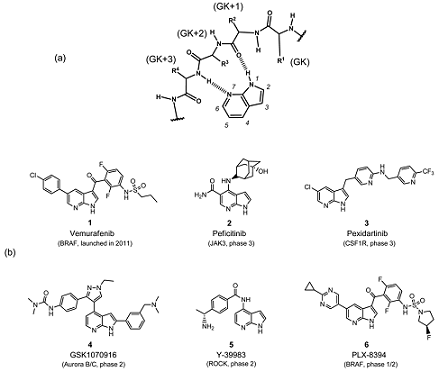
7-Azaindole is a multifunctional molecular fragment with specific pharmacological effects and the most prominent representative of the pyrrolopyridine fragment family. It is already present in the chemical structures of several approved antineoplastic drugs as well as numerous candidate therapies. According to the literature, azaindole and its derivatives possess a wide range of biological activities including kinase inhibitors, cytotoxic agents, anti-angiogenic activity, CRTh2 receptor antagonists, melanin agonists, nicotine agonists, efficacy against Alzheimer's disease, cytokinin analogues, Orai inhibitors against asthma and chemokine receptor-2 (CCR2) antagonists. Therefore, the development of drugs for the treatment of cancer and other diseases based on the structure of Azaindole is of great importance.

Uses of 7-Azaindole
7-Azaindole is primarily used in the pharmaceutical field as a key component of cancer treatment drugs and many other disease medications. 7-Azaindole is used as a raw material for the synthesis of 7-azatryptophan, which is used to inhibit the growth of tobacco and carrot cells. Its derivatives have been effectively tested as potential agrochemicals. 7-Azaindole and its derivatives are also mostly used in pharmacophore studies for the development of new potential active ingredient candidates.
Synthesis of 7-Azaindole
7-Azaindole was synthesised by a Rh(III) catalyst coupling 2-aminopyridine and alkynyl groups. The catalytic process requires the assistance of an external silver oxidant, which is thought to regenerate the catalyst and improve turnover efficiency.
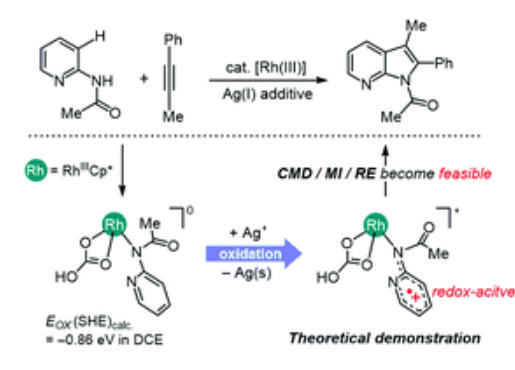
Ag can oxidise a variety of neutral Rh(III) intermediates encountered at different stages of catalysis. One of the catalytically relevant species is the cationic Rh(III)- pyridine complex, which undergoes C-H activation of pyridine and couples the internal alkyne substrate to the pyridine ligand to form the desired 7-azaindole product. Furthermore, calculations show that oxidation also accelerates the reaction steps, including C-H activation, 1,2-alkyne insertion and reductive elimination via co-metallation deprotonation (CMD), thus highlighting the role of Ag as a catalytic promoter of the oxidation-induced reactivity of Rh catalysts in the synthesis of 7-azaindoles.
References:
[1] NEHA SHARMA Anurag. 7-Azaindole Analogues as Bioactive Agents and Recent Results.[J]. Mini reviews in medicinal chemistry, 2019, 19 9. DOI:10.2174/1389557518666180928154004.[2] LEANDRO MARCOS SANTOS Nelson J F da S. Current Fragment-to-lead Approaches Starting from the 7-azaindole: The Pharmacological Versatility of a Privileged Molecular Fragment.[J]. Current topics in medicinal chemistry, 2023, 23 22. DOI:10.2174/1568026623666230718100541.
[3] RYU H, PUDASAINI B, CHO D, et al. Oxidatively induced reactivity in Rh(iii)-catalyzed 7-azaindole synthesis: insights into the role of the silver additive?[J]. Chemical Science, 2022, 36: Page 10585 to 10972. DOI:10.1039/D2SC01650H.
Related articles And Qustion
Lastest Price from 7-Azaindole manufacturers

US $9.85/KG2025-04-21
- CAS:
- 271-63-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 m
US $0.00-0.00/KG2025-04-04
- CAS:
- 271-63-6
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1Ton