D-Threonine: An Important Unnatural Amino acids
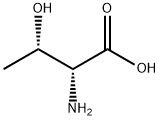
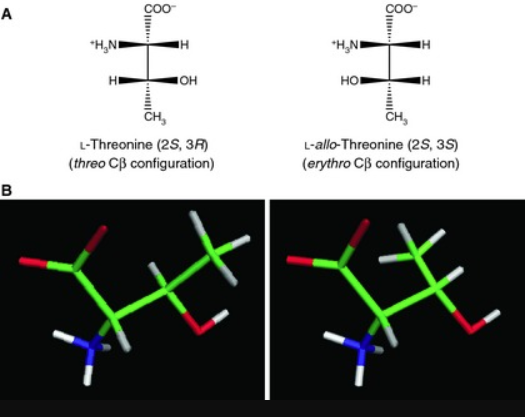
Unnatural amino acids have gained a growing attention in chemical industry owing to their versatile utilities as chiral intermediates for a wide range of pharmaceutical drugs. D-Threonine is one of the important unnatural amino acids and is used as a chiral building block of peptidomimetic drugs for treatment of peripheral opioids side effects and for analgesic applications. Threonine bears two chiral centers, leading to four stereo-chemical isomers among which (2S, 3R) and (2R, 3S) configurations correspond to L- and D-enantiomers of threonine, respectively. In contrast to l-threonine which is mass-produced by microbial fermentation for animal feed applications, such a fermentative production is not yet available for D-threonine.
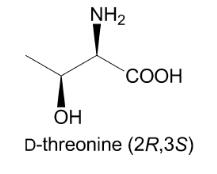
D-Threonine is an optically active form of threonine having D-configuration. It has a role as a Saccharomyces cerevisiae metabolite. It is a threonine and a D-alpha-amino acid. It is a conjugate base of a D-Threoninium. It is a conjugate acid of a D-Threoninate. It is an enantiomer of a L-Threonine. It is a tautomer of a D-Threonine zwitterion.
The stereochemical complexity of threonine renders stereos-elective organic synthesis of D-threonine impracticable for an industrial scale-up due to low conversion and/or coproduction of stereochemical impurities. In contrast, organic synthesis of DL-threonine with a negligible level of diastereomeric impurities has been well established. This has spurred development of chiral resolution of dl-threonine by preferential crystallization, which requires several successive crystallizations to achieve a high enantiopurity.
Owing to the presence of two chiral centers, a synthetic protocol, either through chemocatalysisor biocatalysis, has not yet been available for one-step preparation of stereochemically pure D-threonine in terms of enantiomeric and diastereomeric excesses (i.e., both >99%). Sang-Woo Han et al. reported a facile production of D-threonine using threonine deaminase (TD) via kinetic resolution of DL-threonine that could be readily prepared by conventional organic synthesis. TD catalyzed the dehydration/deamination of L-threonine, leading to generation of 2-oxobutyrate and ammonia. In contrast to mild substrate inhibition of the TD activity by L-threonine, D-threonine turned out to be a strong inhibitor. In addition to the enzyme inhibitions by both enantiomers of threonine, cell lysis observed during small-scale kinetic resolutions of ≥ 1 M DL-threonine led us to carry out a preparative-scale reaction at 500 mM racemic substrate.
References
[1] J.S. Ma, Chim. Oggi 21 (2003) 65–68.
[2] M. Breuer, K. Ditrich, T. Habicher, B. Hauer, M. Keßeler, R. Stürmer, et al.,
Angew. Chem. Int. Ed. 43 (2004) 788–824.
[3] A.S. Bommarius, M. Schwarm, K. Drauz, Chimia 55 (2001) 50–59.
[4] S. Servi, D. Tessaro, G. Pedrocchi-Fantoni, Coord. Chem. Rev. 252 (2008)715–726.
[5] A. Lipkowski (2014) U.S. Patent 303095A1.
[6] A.W. Lipkowski, D. Carr, A. Misicka-Kesik, P. Kosson, A. Klinowiecka (2010)U.S. Patent 273715A1.
You may like
Related articles And Qustion
See also
Lastest Price from D-Threonine manufacturers

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 632-20-2
- Min. Order:
- 1KG
- Purity:
- 98%-101%
- Supply Ability:
- 500KGS

US $999.00-800.00/kg2025-04-21
- CAS:
- 632-20-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000