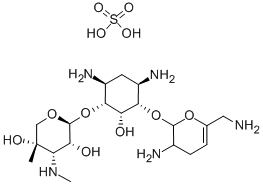
Sisomicin
- русский язык имя
- английское имяSisomicin
- CAS №32385-11-8
- CBNumberCB9875243
- ФормулаC19H37N5O7
- мольный вес447.53
- EINECS251-018-2
- номер MDLMFCD00865123
- файл Mol32385-11-8.mol
| Температура плавления | 80-93°C |
| Температура кипения | 556.56°C (rough estimate) |
| плотность | 1.2777 (rough estimate) |
| показатель преломления | 1.7600 (estimate) |
| температура хранения | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| растворимость | Aqueous Acid (Slightly), DMSO (Slightly), Methanol (Slightly), Water (Slightly, |
| форма | Solid |
| пка | 13.29±0.70(Predicted) |
| цвет | Pale Yellow to Yellow |
| FDA UNII | X55XSL74YQ |
| Код УВД | J01GB08 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H360:Может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
H332:Вредно при вдыхании.
H312:Вредно при попадании на кожу.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P312:Обратиться за медицинской помощью при плохом самочувствии.
P363:Перед повторным использованием выстирать загрязненную одежду.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Sisomicin химические свойства, назначение, производство
Описание
Sisomicin was found in the culture broth of Micromonospora inyoensis by Schering-Plough Co. in 1970, following the discovery of gentamicin by the same research group. The structure and activity of sisomicin are very similar to those of gentamicin C1a, the major component of the gentamicin complex. Sisomicin shows stronger bacterial activity and lower renal and ototoxicity than gentamicin C1a .Химические свойства
Off-White SolidИспользование
Sisomicin is an Antibacterial. Gentamicin-like aminoglycoside antibiotic; has broad spectrum antibiotic activity.Антимикробная активность
A fermentation product of Micromonospora inyoensis. A dehydro derivative of gentamicin C1a, supplied as the sulfate salt.It is virtually identical to gentamicin in activity and pharmacokinetic behavior. An intramuscular dose of 1–1.5 mg/kg achieves a peak plasma concentration of 1.5–9.0 mg/L after 0.5–1 h. It is widely distributed in body water, but concentrations in CSF are low, even in the presence of inflammation. The plasma half-life is 2.5 h and protein binding is <10%.
It is eliminated almost completely over 24 h in the glomerular filtrate. Excretion decreases proportionately with renal impairment and because of the virtual identity of the behavior of the two compounds, a gentamicin nomogram can be used to adjust dosage. About 40% of the dose is eliminated during a 6-h dialysis period, during which the elimination half-life falls to about 8 h.
Mild and reversible impairment of renal function occurs in about 5% of patients. Nephrotoxicity is more likely to be seen in those with pre-existing renal disease or treated concurrently with other potentially nephrotoxic drugs. Ototoxicity mainly affecting vestibular function has been found in about 1% of patients. Neuromuscular blockade and other effects common to aminoglycosides including rashes, paresthesiae, eosinophilia and abnormal liver function tests have been described.
Its uses are identical to those of gentamicin, which it closely resembles. It is of limited availability.
Общее описание
This aminoglycoside antibiotic is produced by Micromonospora inyoensis. Sisomicin is very similar to gentamicin in its antimicrobial spectrum and all other properties. It is as active as gentamicin against all Enterobacteriaceae . Against Pseudomonas aeruginosa, sisomicin is more active than gentamicin, but not as active as tobramycin. There is almost complete cross-resistance between gentamicin and sisomicin with most Gram-negative bacilli. The reason for this is that sisomicin, like gentamicin, is affected by at least eight of the plasmid-coded modifying enzymes, which can be produced by Gram-negative bacilli). On the other hand, sisomicin is active against some organisms which resist gentamicin by nonenzymatic mechanisms. Sisomicin does not offer significant advantages over gentamicin. It has had limited clinical trials, and has been available commercially in Europe as a sulfate, but not in the USA, UK, and Australia. The drug dosage of sisomicin (3–6 mg/kg/day), its methods of administration, and pharmacokinetics, are similar to those of gentamicin. The toxicity of these two drugs is also probably about the same. Results of treatment of conditions, such as urinary tract infections or Gramnegative bacillary septicemias, have, in general, been similar to what would be expected from an identical gentamicin regimen.Sisomicin запасные части и сырье
Sisomicin поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| 16314854226; +16314854226 |
United States | 19741 | 58 | |
| +86-057181025280; +8617767106207 |
China | 49734 | 58 | |
| +undefined18051384581 | China | 3135 | 58 | |
| +8618327326525 | China | 8467 | 58 |