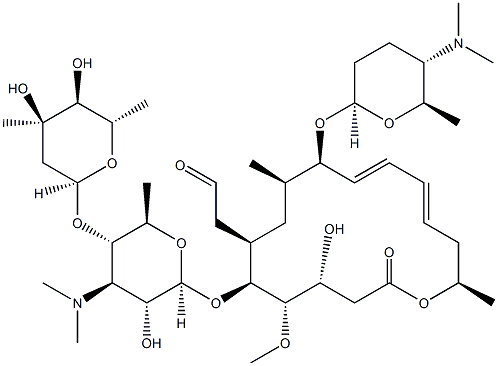
Спирамицин
- английское имяSpiramycin
- CAS №8025-81-8
- CBNumberCB0396400
- ФормулаC43H74N2O14
- мольный вес843.05
- EINECS232-429-6
- номер MDLMFCD01314545
- файл Mol8025-81-8.mol
химическое свойство
| Температура плавления | 126-128 °C |
| Температура кипения | 914℃ |
| альфа | D20 -80° (methanol) |
| показатель преломления | 81 ° (C=2, 10% AcOH) |
| Fp | >110°(230°F) |
| температура хранения | 2-8°C |
| растворимость | ethanol: 50 mg/mL, clear to slightly hazy, light-yellow |
| форма | powder |
| цвет | white to light yellow |
| оптическая активность | [α]/D -85 to -80° in water (Specific rotation (dry basis)) |
| Растворимость в воде | Soluble in methanol. Slightly soluble in water |
| Мерк | 14,8752 |
| Справочник по базе данных CAS | 8025-81-8 |
| Рейтинг продуктов питания EWG | 4 |
| FDA UNII | 71ODY0V87H |
| Код УВД | J01FA02 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36-24/25 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | WG9400000 | |||||||||
| F | 10 | |||||||||
| кода HS | 29419090 | |||||||||
| Банк данных об опасных веществах | 8025-81-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in rats (mg/kg): 9400 orally; 1000 s.c.; 170 i.v. (Sous) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
оператор предупредительных мер
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P403:Хранить в хорошо вентилируемом месте.
Спирамицин химические свойства, назначение, производство
Описание
Spiramycin was found in the culture broth of Streptomyces ambofaciens by Rhone Poulenc in 1954 and its acetate was synthesized by Kyowa Hakko Kogyo Co. in 1965. This antibiotic consists of three closely related macrolide components, I, II, and III, whose ratio is 2 : 1 : 1. Spiramycin shows almost the same antimicrobial spectrum and activity as the other macrolides, but its acetate, acetylspiramycin, has much better pharmacokinetic properties and activity in vivo.Химические свойства
An antibiotic substance.Использование
Spiramycin I (foromacidin A) is the major analogue of a complex of 16-membered macrocyclic lactones produced by S. ambofaciens and S. spiramyceticus that have broad spectrum antibiotic activity. Spiramycins are unusual among the macrocyclic lactones in that they contain two basic sugars. Spiramycin complex has been used in both human and animal health but its use has not been widespread.Антимикробная активность
Enterobacteriaceae are resistant. Spiramycin is also active against anaerobic species: Actinomyces israelii (MIC 2–4 mg/L), Cl. perfringens (MIC 2–8 mg/L) and Bacteroides spp. (MIC 4–14 mg/L). It is also active against Tox. gondii.Опасность
Poison; moderately toxic; teratogen; muta- gen; can cause hypermotility, diarrhea, nausea, or vomiting.Фармацевтические приложения
A fermentation product of Streptomyces ambofaciens, composed of several closely related compounds. Spiramycin 1 is the major component (c. 63%); spiramycins 2 and 3 are the acetate and monopropionate esters, respectively. It is available for oral administration and as spiramycin adipate for intravenous infusion. Spiramycins are relatively stable in acid conditions. A derivative, acetylspiramycin, is available in Japan.Фармакокине?тика
Oral absorption: VariableCmax 1 g oral: 2.8 mg/L after 2 h
Plasma half-life:4–8h
Volume of distribution :383 L
Plasma protein binding :15%
In healthy volunteers given 2 g orally followed by 1 g every 6 h, peak plasma levels were 1.0–6.7 mg/L. After 1 g orally the AUC was 10.8 mg.h/L, with an apparent elimination halflife of 2.8 h. It is widely distributed in the tissues. It does not reach the CSF. Levels 12 h after a dose of 1 g were 0.25 mg/L in serum, 5.3 mg/L in bone and 6.9 mg/L in pus. Levels of 10.6 mg/L have been found 4 h after dosing in saliva, and concentrations at least equal to those in the serum are seen in bronchial secretions. A concentration of 27 mg/g was found in prostate tissues after repeated dosage. Only 5–15% is recovered from the urine. Most is metabolized, but significant quantities are eliminated via the bile, in which concentrations up to 40 times those in the serum may be found.
Побочные эффекты
Spiramycin is generally well tolerated, the most common adverse reactions being gastrointestinal disturbances, notably abdominal pain, nausea and vomiting, rashes and sensitization following contact.Спирамицин запасные части и сырье
Спирамицин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +86-027-59207850 | China | 5972 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +86-0571-28186870; +undefined8613073685410 |
China | 1015 | 58 | |
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | |
| +8618092446649 | China | 1143 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 |