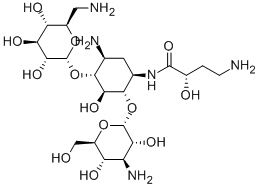
Амикацин
- английское имяAMIKACIN
- CAS №37517-28-5
- CBNumberCB8146049
- ФормулаC22H43N5O13
- мольный вес585.6
- EINECS253-538-5
- номер MDLMFCD00883675
- файл Mol37517-28-5.mol
химическое свойство
| Температура плавления | 203℃ |
| альфа | D23 +99° (c = 1.0 in water) |
| Температура кипения | 642.23°C (rough estimate) |
| плотность | 1.3764 (rough estimate) |
| показатель преломления | 1.7500 (estimate) |
| температура хранения | 2-8°C |
| растворимость | H2O: 50 mg/mL, clear, colorless |
| пка | pKa 8.1 (Uncertain) |
| форма | solid |
| цвет | white to off-white |
| оптическая активность | 99(c 1.0) |
| Растворимость в воде | Soluble in water (partly). |
| Мерк | 13,404 |
| БРН | 1445422 |
| Стабильность | Hygroscopic |
| FDA UNII | 84319SGC3C |
| Словарь онкологических терминов NCI | amikacin |
| Код УВД | D06AX12,J01GB06,S01AA21 |
| Система регистрации веществ EPA | Amikacin (37517-28-5) |
больше
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36-24/25 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | WK1955000 | |||||||||
| F | 10-34 | |||||||||
| кода HS | 29419090 | |||||||||
| Банк данных об опасных веществах | 37517-28-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in mice of solns pH 6.6, pH 7.4 (mg/kg): 340, 560 i.v. (Kawaguchi) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
Амикацин химические свойства, назначение, производство
Описание
Amikacin is made semisynthetically from kanamycin A. Interestingly, the L-hydroxyaminobutyryl amide (HABA) moiety attached to N-3 inhibits adenylation and phosphorylation in the distant amino sugar ring (at C-2′and C-3′), even though the HABA substituent is not where the enzymatic reaction takes place. This effect is attributed to decreased binding to the R factor–mediated enzymes.Химические свойства
white crystalline powderИспользование
Amikacin is a semi-synthetic derivative of kanamycin. It is much less sensitive to the enzymes that inactivate aminoglycoside antibiotics. The spectrum is similar to that of gentamicin. Amikacin principally finds use in the treatment of infections arising from bacteria that are resistant to gentamicin and/or tobramycin.Определение
ChEBI: An amino cyclitol glycoside that is kanamycin A acylated at the N-1 position by a 4-amino-2-hydroxybutyryl group.Антимикробная активность
Among other organisms, Acinetobacter, Alkaligenes, Campylobacter, Citrobacter, Hafnia, Legionella, Pasteurella, Providencia, Serratia and Yersinia spp. are usually susceptible in vitro. Stenotrophomonas maltophilia, many nonaeruginosa pseudomonads and Flavobacterium spp. are resistant. M. tuberculosis (including most streptomycin-resistant strains) and some other mycobacteria (including M. fortuitum and the M. avium complex) are susceptible; most other mycobacteria, including M. kansasii, are resistant. Nocardia asteroides is susceptible.It exhibits typical aminoglycoside characteristics, including an effect of divalent cations on its activity against Ps. aeruginosa analogous to that seen with gentamicin and synergy with β-lactam antibiotics.
Приобретенная устойчивость
Amikacin is unaffected by many of the modifying enzymes that inactivate gentamicin and tobramycin and is consequently active against staphylococci, enterobacteria and Pseudomonas that owe their resistance to the production of those enzymes. However, AAC(6′), ANT(4′) and some forms of APH(3′) can confer resistance; because these enzymes generally do not confer gentamicin resistance, amikacin-resistant strains can be missed in routine susceptibility tests when gentamicin is used as the representative aminoglycoside.There have been reports of resistance arising during treatment of infections due to Serratia spp. and Ps. aeruginosa. Outbreaks of infection with multiresistant strains of enterobacteria and Ps. aeruginosa have occurred after extensive use, particularly in burns units. Bacteria that owe their resistance to the expression of ANT(4′) have been described in Staph. aureus, coagulase-negative staphylococci, Esch. coli, Klebsiella spp. and Ps. aeruginosa. In E. faecalis, resistance to penicillin– aminoglycoside synergy has been associated with plasmidmediated APH(3′). Resistance in Gram-negative organisms is usually caused by either reduced accumulation of the drug or, more commonly, by the aminoglycoside-modifying enzymes AAC(6′) or AAC(3)-VI. The latter enzyme is usually found in Acinetobacter spp., but has also been found, encoded by a transposon, in Prov. stuartii. One type of AAC(6) is chromosomally encoded by Ser. marcescens, though not usually expressed.
The prevalence of resistance to amikacin remains low (<5%) in many countries but can change rapidly with increased usage of the drug. However, the spread of extended spectrum β-lactamases belonging to the TEM and SHV families may result in an increase in amikacin resistance that is not associated with use, since most strains that produce such enzymes also produce AAC(6′).
Общее описание
Amikacin was synthesized by Kawaguchi et al. of the Bristol-Banyu Research Institute in 1970 starting with kanamycin and the acyl moiety of butirosin. Its design is based on knowledge of the mechanisms of bacterial resistance to kanamycin and related compounds in which the 3 -hydroxyl group of the antibiotic is phosphorylated enzymatically. The acyl moiety in butirosin prevents this enzymatic inactivation.Фармакокине?тика
Cmax 7.5 mg/kg intramuscular: c. 30 mg/L after 1 h500 mg 30-min infusion: 35–50 mg/L end infusion
15 mg/kg 30-min infusion: >50 mg/L after 1 h
Plasma half-life: 2.2 h
Volume of distribution: 0.25–0.3 L/kg
Plasma protein binding: 3–11%
It is readily absorbed after intramuscular administration. Rapid intravenous injection of 7.5 mg/kg produced concentrations in excess of 60 mg/L shortly after injection.
Most pharmacokinetic parameters follow an almost linear correlation when the once-daily doses (15 mg/kg) are compared with the traditional 7.5 mg/kg twice daily. In patients on CAPD, there was no difference in mean peak plasma concentration or volume of distribution whether the drug was given intravenously or intraperitoneally. However, in patients with significant burn injuries, doses should be increased to 20 mg/kg.
In infants receiving 7.5 mg/kg by intravenous injection, peak plasma concentrations were 17–20 mg/L. No accumulation occurred on 12 mg/kg per day for 5–7 days. There was little change in the plasma concentration or the half-life (1.7 and 1.9 h) on the third and seventh days of a period over which 150 mg/m2 was infused over 30 min every 6 h. When the dose was raised to 200 mg/m2 the concentration never fell below 8 mg/L. The plasma half-life was longer in babies of lower birth weight and was still 5–5.5 h in babies aged 1 week or older. The importance of dosage control in the neonate is emphasized by the findings that there is an inverse relationship between post-conception age and plasma elimination half-life, though in extremely premature babies the weight of the child is also a significant predictor of half-life.
Клиническое использование
Severe infection (including septicemia, neonatal sepsis, osteomyelitis, septic arthritis, respiratory tract, urinary tract, intra-abdominal, peritoneal and soft tissue infections) caused by susceptible micro-organisms Sepsis of unknown origin (combined with a β-lactam or anti-anaerobe agent as appropriate).Mycobacterial infection
Amikacin is principally used for the treatment of infections caused by organisms resistant to other aminoglycosides because of their ability to degrade them. Peak concentrations on 15 mg/kg once daily administration should exceed 45 mg/L, and trough concentration of <5 mg/L should be maintained to achieve therapeutic effects.
Профиль безопасности
Poison by intravenous,intraperitoneal, and intramuscular routes. Moderately toxicby intraperitoneal route. An experimental teratogen. Whenheated to decomposition it emits toxic fumes of NOx.Амикацин запасные части и сырье
сырьё
1of2
запасной предмет
Амикацин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 025-83697070 | CHINA | 3009 | 60 | |
| +8615102730682 | CHINA | 566 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 0086-13720134139 | CHINA | 965 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +8615531157085 | China | 8804 | 58 |