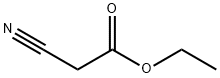
Этил цианоацета
- английское имяEthyl cyanoacetate
- CAS №105-56-6
- CBNumberCB9852660
- ФормулаC5H7NO2
- мольный вес113.11
- EINECS203-309-0
- номер MDLMFCD00001940
- файл Mol105-56-6.mol
| Температура плавления | -22 °C (lit.) |
| Температура кипения | 208-210 °C (lit.) |
| плотность | 1.063 g/mL at 25 °C (lit.) |
| плотность пара | 3.9 (vs air) |
| давление пара | 1 mm Hg ( 67.8 °C) |
| показатель преломления | n |
| Fp | >230 °F |
| температура хранения | Store below +30°C. |
| растворимость | 20g/l |
| форма | Liquid |
| пка | 3.19±0.10(Predicted) |
| цвет | Clear |
| Растворимость в воде | 20 g/L (20 ºC) |
| Мерк | 14,3786 |
| БРН | 605871 |
| Диэлектрическая постоянная | 26.9(20℃) |
| LogP | -0.119-1.05 at 23-25℃ and pH6.1 |
| Surface tension | 70.2mN/m at 1.034g/L and 23℃ |
| Справочник по базе данных CAS | 105-56-6(CAS DataBase Reference) |
| FDA UNII | X9N006U0F8 |
| Справочник по химии NIST | Acetic acid, cyano-, ethyl ester(105-56-6) |
| Система регистрации веществ EPA | Ethyl cyanoacetate (105-56-6) |
| UNSPSC Code | 12352108 |
| NACRES | NA.22 |
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 20/21/22-36/38 | |||||||||
| Заявления о безопасности | 36/37-37/39-26 | |||||||||
| РИДАДР | 3276 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | AG4110000 | |||||||||
| F | 10 | |||||||||
| Температура самовоспламенения | 460 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2926 90 70 | |||||||||
| Банк данных об опасных веществах | 105-56-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: > 2000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
Этил цианоацета химические свойства, назначение, производство
Описание
Ethyl cyanoacetate is the ethyl ester of cyanoacetic acid. Ethyl cyanoacetate hydrolizes rapidly under neutral and alkaline conditions to cyanoacetic acid and ethanol (and so it does under most physiological and environmental conditions), while in acid pH the half life is considerably longer.Knoevenagel condensation of ethyl cyanoacetate with aldehyde is reported. Microwave enhanced Knoevenegal condensation reaction of ethyl cyanoacetate with an aldehyde, P2O5, piperidine and chlorobenzene is reported.
Химические свойства
Ethyl cyanoacetate is a colorless to straw colored liquid with a mild pleasant odorИспользование
Reagent used in labelled pyrimidine and purine synthesis. Ethyl cyanoacetate is an ester. It may be used in the synthesis of ethyl glyoxylate.It was used to investigate the Knoevenagel condensation reactions in microreactor using zeolite catalysts obtained by grafting amino groups onto NaX and CsNaX zeolites.Подготовка
Ethyl cyanoacetate can be prepared by the action of sodium or potassium cyanide on ethyl chloroacetate, and by the action of sodium cyanide on sodium chloroacetate, followed by esterification.Общее описание
A colorless liquid. Denser than water. Contact may irritate skin, eyes and mucous membranes. Flash point 210°F. May be toxic by ingestion. Used to make other chemicals.Реакции воздуха и воды
Slightly soluble in water.Профиль реактивности
Ethyl cyanoacetate is both a nitrile and an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids.Опасность
Toxic by ingestion and inhalation.Угроза здоровью
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.Профиль безопасности
oison by ingestion. Moderately toxic by intraperitoneal and subcutaneous routes. Combustible when exposed to heat or flame; can react with oxidzing materials. Wdl react with water or steam to produce toxic and flammable vapors. To fight fire, use CO2, dry chemical. When heated to decomposition or on contact with acid or acid fumes it emits highly toxic fumes of CN-. See also NITRILES.Возможный контакт
A nitrile used to manufacture dyes, pharmaceuticals, and other chemicals.Перевозки
UN3276 Nitriles, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required, Potential Inhalation Hazard (Special Provision 5).Методы очистки
Shake the ester several times with aqueous 10% Na2CO3, wash it well with water, dry with Na2SO4 and fractionally distil it. [Beilstein 2 IV 1889.]Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, and reducing agents. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids. Reacts with moisture, water, and steam, forming toxic fumes.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.использованная литература
[1] Wohlfarth, C. “Viscosity of ethyl cyanoacetate.” 1900. 0.、[2] A. C. Cope, E. M. Hancock. “Ethyl (1‐Ethylpropylidene)cyanoacetate.” Organic Syntheses 38 1 (2003): 46–46.
[3] Ismailov, V.M. et al. “SYNTHESIS BASED ON ETHYL CYANOACETATE.” Kimya probleml?ri 86 1 (2023): 0.
[4] T.R. Kasturi, A. Srinivasan. “A revised structure for the condensation product of 2-carbethoxycyclopentanone and ethyl cyanoacetate.” Tetrahedron 22 8 (1966): Pages 2575-2580.
[5] Akram Ranjbar Derranji, Mohammad Anary-Abbasinejad. “An efficient synthesis of 4,5-disubstituted 1,2,3-triazoles by three-component reaction between sodium azide, ethyl cyanoacetate or malonitrile and arylglyoxals.” Synthetic Communications 54 6 (2024): Pages 471-477.
Этил цианоацета запасные части и сырье
сырьё
1of2
запасной предмет
- 5-ЦИАНО-6-ГИДРОКСИ-4-МЕТОКСИМЕТИЛ-2-МЕТИЛПИРИДИН
- ЭТИЛ-2-ЦИАНО-3-МЕТИЛ-2-ПЕНТЕНОАТ
- Этил 2-амино-4,5,6,7-тетрагидробензо[b]тиофен-3-карбоксила
- Триамтерен
- 2-(4,6-diamino-1,3,5-triazin-2-yl)acetic acid
- Аллопуринол
- 2-Amino-4-trifluoromethylbenzonitrile
- 5-AMINO-4-CYANO-3-METHYL-THIOPHENE-2-CARBOXYLIC ACID ETHYL ESTER
- 1-ETHYL-1-METHYLSUCCINIC ACID
- Disperse Yellow S-3GL
- 6-AMINO-2-METHYLTHIO-3-METHYLURACIL
- 1,2,3,5-ТЕТРАГИДРО-8-ТИА-5,7-ДИАЗА-ЦИКЛОПЕНТА[А]ИНДЕН-4-ОН
- 2-АМИНО-4,6-ДИМЕТИЛ-3-ПИРИДИНКАРБОКСАМИД
- 6-аминоурацила
- 2-ХЛОРО-4-МЕТИЛХИНОЛИН-3-КАРБОНИТРИЛ
- Этил 2-амино-4,5-диметил-3-тиофенкарбоксила
- 6-AMINO-2-MERCAPTO-PYRIMIDIN-4-OL
- Ethyl α-cyanoacrylate instantaneous adhesive
- ЭТИЛОВЫЙ ЭФИР 2-АМИНО-6-МЕТИЛ-4,5,6,7-ТЕТРАГИДРОБЕНЗ[B]ТИОФЕН-3-КАРБОНОВОЙ КИСЛОТЫ
- Ethyl 4-amino-2-(ethylthio)-5-pyrimidinecarboxylate
- 3-Амино-2 ,2-диметил-1-пропанола
- 2-AMINO-5-ISOPROPYL-THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER
- ЭТИЛОВЫЙ ЭФИР 2-АМИНО-4-ЭТИЛ-5-МЕТИЛ-ТИОФЕН-3-КАРБОКСИЛОВОЙ КИСЛОТЫ
- ETHYL(Z)-2-CYANO-3-ETHOXY-2-PROPENOATE
- Гидрохлорид Амилорид дигидра
- 5-AMINO-3-METHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID ETHYL ESTER
- 6-амино-1-метилурацил
- 2-ФОРМАМИДИНО-2-ФЕНИЛДИАЗОАЦЕТАМИДА ГИДРОХЛОРИД
- 1-Cyclohexyl-1,2,3,4-tetrahydro-2,4-dioxopyrimidine-5-carbonitrile ,97%
- 6-HYDROXY-2,4,5-TRIAMINOPYRIMIDINE
1of8
Этил цианоацета поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-531-88989536 +86-15508631887 |
China | 2958 | 58 |
Этил цианоацета Обзор)
1of4