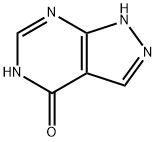
Аллопуринол
- английское имяAllopurinol
- CAS №315-30-0
- CBNumberCB1181254
- ФормулаC5H4N4O
- мольный вес136.11
- EINECS206-250-9
- номер MDLMFCD00599413
- файл Mol315-30-0.mol
химическое свойство
| Температура плавления | >300 °C (lit.) |
| Температура кипения | 250.36°C (rough estimate) |
| плотность | 1.4295 (rough estimate) |
| показатель преломления | 1.8500 (estimate) |
| температура хранения | 15-25°C |
| растворимость | 1 M NaOH: soluble50mg/mL, clear to very slightly hazy, colorless to faintly yellow |
| форма | Powder |
| пка | 10.2(at 25℃) |
| цвет | White or almost white |
| Биологические источники | synthetic (organic) |
| Растворимость в воде | 0.35 g/L (25 ºC) |
| Мерк | 14,279 |
| BCS Class | 3,1 |
| ИнЧИКей | OFCNXPDARWKPPY-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 315-30-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| Словарь онкологических терминов NCI | allopurinol |
| FDA UNII | 63CZ7GJN5I |
| Словарь наркотиков NCI | Lopurin |
| Код УВД | M04AA01,M04AA51 |
| Справочник по химии NIST | Allopurinol(315-30-0) |
| Система регистрации веществ EPA | Allopurinol (315-30-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
больше
| Коды опасности | T,Xi,Xn | |||||||||
| Заявления о рисках | 25-43-36/37/38-20/21/22 | |||||||||
| Заявления о безопасности | 28-36/37-45-36/37/39-26-24-36 | |||||||||
| РИДАДР | UN 2811 6.1/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | UR0785000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29335990 | |||||||||
| Банк данных об опасных веществах | 315-30-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 oral in mouse: 78mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310+P330:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
Аллопуринол химические свойства, назначение, производство
Химические свойства
White to Off-White SolidИспользование
Allopurinol does not reduce serum uric acid levels by increasing renal uric acid excretion; instead it lowers plasma urate levels by inhibiting the final steps in uric acid biosynthesis.Uric acid in humans is formed primarily by xanthine oxidase-catalyzed oxidation of hypoxanthine and xanthine to uric acid. Allopurinol (8) and its primary metabolite, alloxanthine (9) [CAS: 2465-59-0], are inhibitors of xanthine oxidase. Inhibition of the last two steps in uric acid biosynthesis by blocking xanthine oxidase reduces the plasma concentration and urinary excretion of uric acid and increases the plasma levels and renal excretion of the more soluble oxypurine precursors. Normally, in humans the urinary purine content is almost solely uric acid; treatment with allopurinol results in the urinary excretion of hypoxanthine, xanthine, and uric acid, each with its independent solubility. Lowering the uric acid concentration in plasma below its limit of solubility facilitates the dissolution of uric acid deposits. The effectiveness of allopurinol in the treatment of gout and hyperuricemia that results from hematogical disorders and antineoplastic therapy has been demonstrated.
Показания
Allopurinol (Zyloprim) is the drug of choice in the treatment of chronic tophaceous gout and is especially useful in patients whose treatment is complicated by renal insufficiency.Общее описание
Odorless tasteless white microcrystalline powder.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Allopurinol is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Allopurinol darkens above 572° F, and at an indefinite high temperature, Allopurinol chars and decomposes. At 221° F, maximum stability occurs at pH 3.1- 3.4. Allopurinol decomposes in acidic and basic solutions.Пожароопасность
Flash point data for Allopurinol are not available; however, Allopurinol is probably combustible.Механизм действия
Allopurinol, in contrast to the uricosuric drugs, reduces serum urate levels through a competitive inhibition of uric acid synthesis rather than by impairing renal urate reabsorption. This action is accomplished by inhibiting xanthine oxidase, the enzyme involved in the metabolism of hypoxanthine and xanthine to uric acid. After enzyme inhibition, the urinary and blood concentrations of uric acid are greatly reduced and there is a simultaneous increase in the excretion of the more soluble uric acid precursors, xanthine and hypoxanthine.Allopurinol itself is metabolized by xanthine oxidase to form the active metabolite oxypurinol, which tends to accumulate after chronic administration of the parent drug.This phenomenon contributes to the therapeutic effectiveness of allopurinol in long-term use. Oxypurinol is probably responsible for the antigout effects of allopurinol. Oxypurinol itself is not administered because it is not well absorbed orally.
Фармакокине?тика
Allopurinol was synthesized in 1956 as part of a study of purine antagonists. It is well absorbed on oral administration, with peak plasma concentrations appearing within 1 hour. Decreases of uric acid can be observed within 24 to 48 hours. Excretion of allopurinol and its metabolite occurs primarily in the urine, with approximately 20% of a dose being excreted in the feces.Клиническое использование
Allopurinol is especially indicated in the treatment of chronic tophaceous gout, since patients receiving it show a pronounced decrease in their serum and urinary uric acid levels. Because it does not depend on renal mechanisms for its efficacy, allopurinol is particularly beneficial for patients who already have developed renal uric acid stones, patients with excessively high urate excretion (e.g., above 1,200 mg in 24 hours), patients with a variety of blood disorders (e.g., leukemia, polycythemia vera), patients with excessive tophus deposition, and patients who fail to respond well to the uricosuric drugs.Allopurinol also inhibits reperfusion injury. This injury occurs when organs that either have been transplanted or have had their usual blood perfusion blocked are reperfused with blood or an appropriate buffer solution. The cause of this injury is local formation of free radicals, such as the superoxide anion, the hydroxyl free radical, or peroxynitrite. These substances are strong oxidants and are quite damaging to tissues.
Побочные эффекты
Common toxicities associated with allopurinol administration include a variety of skin rashes, gastrointestinal upset, hepatotoxicity, and fever. These reactions are often sufficiently severe to dictate termination of drug therapy. It is advised that therapy not be initiated during an acute attack of gouty arthritis. As with the uricosuric drugs, therapy with allopurinol should be accompanied both by a sufficient increase in fluid intake to ensure water diuresis and by alkalinization of the urine. Prophylactic use of colchicine also helps to prevent acute attacks of gout that may be brought on during the initial period of allopurinol ingestion.Профиль безопасности
Human poison by ingestion. Poison experimentally by intraperitoneal and subcutaneous routes. An experimental teratogen. Human systemic effects by ingestion: blood leukopenia, dermatitis, jaundice, muscle weakness, thrombocytopenia. When heated to decomposition it emits toxic fumes of NOx. An FDA proprietary drug used as a xanthine oxidase inhibitor.Метаболизм
Allopurinol is rapidly metabolized via oxidation and the formation of numerous ribonucleoside derivatives. The major oxidation metabolite, alloxanthine or oxypurinol, has a much longer half-life (18–30 hours versus 2–3 hours) than the parent drug and is an effective, although less potent, inhibitor of xanthine oxidase. The longer plasma half-life of alloxanthine results in an accumulation in the body during chronic administration, thus contributing significantly to the overall therapeutic effects of allopurinol.Меры предосторожности
Since allopurinol is metabolized by the hepatic microsomaldrug-metabolizing enzymes, coadministration ofdrugs also metabolized by this system should be donewith caution. Because allopurinol inhibits the oxidationof mercaptopurine and azathioprine, their individualadministered doses must be decreased by as much as75% when they are given together with allopurinol.Allopurinol may also increase the toxicity of other cytotoxicdrugs (e.g., vidarabine). The actions of allopurinolare not antagonized by the coadministration of salicylates.Аллопуринол запасные части и сырье
Аллопуринол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0571-89385087 +8613616530509 |
China | 118 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 988 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +8615350851019 | China | 1001 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 |