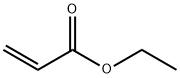
ЭТИЛАКРИЛАТ
- английское имяEthyl acrylate
- CAS №140-88-5
- CBNumberCB8382425
- ФормулаC5H8O2
- мольный вес100.12
- EINECS205-438-8
- номер MDLMFCD00009188
- файл Mol140-88-5.mol
| Температура плавления | −71 °C(lit.) |
| Температура кипения | 99 °C(lit.) |
| плотность | 0.921 g/mL at 20 °C |
| плотность пара | 3.5 (vs air) |
| давление пара | 31 mm Hg ( 20 °C) |
| показатель преломления | n |
| FEMA | 2418 | ETHYL ACRYLATE |
| Fp | 60 °F |
| температура хранения | 2-8°C |
| растворимость | 20g/l |
| форма | Liquid |
| цвет | Clear |
| Запах | Characteristic acrylic odor; sharp, fragrant; acrid; slightly nauseating; sharp, ester type. |
| Порог?обнаружения?запаха? | 0.00026ppm |
| Пределы взрываемости | 1.8-14%(V) |
| Odor Type | plastic |
| Биологические источники | synthetic |
| Вязкость | 0.53 cp (25C) |
| Растворимость в воде | 1.5 g/100 mL (25 ºC) |
| Точка замерзания | 99.8℃ |
| Номер JECFA | 1351 |
| Мерк | 14,3759 |
| БРН | 773866 |
| констант закона Генри | 2.25(x 10-3 atm?m3/mol) at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Пределы воздействия | TLV-TWA 5 ppm (~20 mg/m3) (ACGIH), 25 ppm (~100 mg/m3 (MSHA, NIOSH), TWA skin 25 ppm (100 mg/m3) (OSHA); IDLH 2000 ppm (NIOSH). |
| Стабильность | Stable, but may polymerize upon exposure to light. Highly flammable. Keep cool. Incompatible with oxidizing agents, peroxides and other polymerization initiators. |
| LogP | 1.18 at 25℃ |
| Surface tension | 25.72mN/m at 293.15K |
| Toxicology and Carcinogenesis | Carcinogenesis Studies of Ethyl Acrylate (CASRN 140-88-5) in F344/N Rats and B6C3F1 Mice (Gavage Studies) |
| Вещества, добавляемые в пищу (ранее EAFUS) | ETHYL ACRYLATE |
| Специальный комитет по веществам GRAS | Ethyl acrylate, monomeric (packaging) |
| FDA 21 CFR | 175.105; 175.300; 175.320; 177.1010; 178.3790 |
| Справочник по базе данных CAS | 140-88-5(CAS DataBase Reference) |
| FDA UNII | 71E6178C9T |
| МАИР | 2B (Vol. 39, Sup 7, 71, 122) 2019 |
| Предложение 65 Список | Ethyl Acrylate |
| Справочник по химии NIST | 2-Propenoic acid, ethyl ester(140-88-5) |
| Система регистрации веществ EPA | Ethyl acrylate (140-88-5) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | F,Xn | |||||||||
| Заявления о рисках | 11-20/21/22-36/37/38-43 | |||||||||
| Заявления о безопасности | 9-16-33-36/37 | |||||||||
| РИДАДР | UN 1917 3/PG 2 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | AT0700000 | |||||||||
| F | 8 | |||||||||
| Температура самовоспламенения | 721 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2916 12 00 | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 140-88-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 550 mg/kg LD50 dermal Rabbit 1800 mg/kg | |||||||||
| ИДЛА | 300 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H302+H312:Вредно при проглатывании или при попадании на кожу.
H331:Токсично при вдыхании.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
ЭТИЛАКРИЛАТ химические свойства, назначение, производство
Описание
Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers . It is also a reagent in the synthesis of various pharmaceutical intermediates.Химические свойства
Ethyl acrylate is a colorless liquid. Sharp, acrid odor. The Odor Threshold is 0.00024~0.0009 ppm. 1.5 % soluble in water, miscible with alcohol and oils. Ethyl acrylate absorbs 1.5% water in aqueous media. It polymerizes easily under influence of air, heat or daylight. Fairly stable below 10℃. The polymer form is a transparent, resilient, odorless mass.Физические свойства
Clear, colorless liquid with a penetrating and pungent odor. Leonardos et al. (1969) and Nagata and Takeuchi (1990) reported odor threshold concentrations of 0.47 and 0.26 ppbv, respectively. Experimentally determined detection and recognition odor threshold concentrations were 1.0 μg/m3 (0.24 ppbv) and 1.5 μg/m3 (0.37 ppbv), respectively (Hellman and Small, 1974).Вхождение
Reported found in pineapple, yellow passion fruit and durian (Durio zibethinus).Использование
Ethyl Acrylate is a flavoring agent that is a clear, colorless liquid. its odor is fruity, harsh, penetrating, and lachrymatous (causes tears). it is sparingly soluble in water and miscible in alcohol and ether, and is obtained by chemical synthesis.Подготовка
By esterification of acrylic acid; by heating acetylene with HCl in alcoholic solution in the presence of Ni(CO)4; also from ethyl-3-chloropropionate passed over activated carbon at high temperature.Методы производства
Ethyl acrylate is manufactured via oxidation of propylene to acrolein and then to acrylic acid. The acid is treated with ethanol to yield the ethyl ester .Vinyl chloride reacts at 270 °C at >6895 kPa (68 atm) with ethanol in the presence of a cobalt and palladium catalyst to give ethyl acrylate in a yield of 17% .
Общее описание
A clear colorless liquid with an acrid odor. Flash point 60°F. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently. Auto ignition temperature 721°F (383°C) (NTP). Less dense than water. Vapors heavier than air. Used to make paints and plastics.Реакции воздуха и воды
Highly flammable. Insoluble in water.Профиль реактивности
A flammable liquid, confirmed carcinogen. Ethyl acrylate can react vigorously with oxidizing reagents, peroxides,strong alkalis and polymerization initiators. [NTP] Ethyl acrylate reacts violently with chlorosulfonic acid [Sax, 9th ed., 1996, p. 1515]. When an inhibited monomer was placed in a clear glass bottle exposed to sunlight, exothermic polymerization set in and caused the bottle to burst. The use of brown glass or metal containers and increase in inhibitor concentration (to 200 ppm; tenfold) was recommended [MCA Case History No. 1759]. Ethyl acrylate may polymerize when exposed to light and Ethyl acrylate is subject to slow hydrolysis. Inhibitors do not function in the absence of air. Solutions in DMSO are stable for 24 hours under normal lab conditions. [NTP].Опасность
Toxic by ingestion, inhalation, skin absorption; irritant to skin and eyes. Flammable, dangerous fire and explosion hazard. Possible carcinogen.Угроза здоровью
Ethyl acrylate is a strong irritant to the eyes,skin, and mucous membranes. The liquid orits concentrated solutions can produce skinsensitization upon contact. It is toxic by allroutes of exposure. The toxicity is low inrats and mice and moderate in rabbits. Thetoxic effects from inhalation noted in animalswere congestion of lungs and degenerativechanges in the heart, liver, and kidney. Mon key exposed to 272 ppm for 28 days showedlethargy and weight loss; while exposure to1024 ppm caused death to the animals after2.2 days (Treon et al. 1949). By compari son, guinea pigs died of exposure to about1200 ppm for 7 hours. Ingestion of the liq uid may result in irritation of gastrointestinaltracts, nausea, lethargy, and convulsionsThe LD50 values varied significantly indifferent species of animals. The oral LD50values in rabbits, rats, and mice are in therange 400, 800, and 1800 mg/kg, respectively. Animals administered ethyl acrylateshowed increased incidence of tumors inforestomach. However, there is no evidenceof carcinogenicity caused by this compoundin humans.
Химическая реактивность
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: May occur; exclude moisture, light; avoid exposure to high temperatures; store in presence of air; Inhibitor of Polymerization: 13-17 ppm monomethyl ether of hydroquinone.Профиль безопасности
Confirmed carcinogen with experimental carcinogenic data. Poison by ingestion and inhalation. Moderately toxic by skin contact and intraperitoneal routes. Human systemic effects by inhalation: eye, olfactory, and pulmonary changes. A skin and eye irritant. Characterized in its terminal stages by dyspnea, cyanosis, and convulsive movements. It caused severe local irritation of the gastroenteric tract; and toxic degenerative changes of cardiac, hepatic, renal, and splenic tissues were observed. It gave no evidence of cumulative effects. When applied to the intact skin of rabbits, the ethyl ester caused marked local irritation, erythema, edema, thickening, and vascular damage. Animals subjected to a fairly high concentration of these esters suffered irritation of the mucous membranes of the eyes, nose, and mouth as well as lethargy, dpspnea, and convulsive movements. A substance that migrates to food from packagmg materials. Flammable liquid. A very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Violent reaction with chlorosulfonic acid. To fight fire, use CO2, dry chemical, or alcohol foam. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.Безопасность
It is an acute toxin with an LD50 (rats, oral) of 1020 mg / kg and a TLV of 5 ppm. The International Agency for Research on Cancer stated, "Overall evaluation, Ethyl acrylate is possibly carcinogenic to humans (Group 2B)." The United States Environmental Protection Agency (EPA) states, "Human studies on occupational exposure to ethyl acrylate... have suggested a relationship between exposure to the chemical(s) and colorectal cancer, but the evidence is conflicting and inconclusive. In a study by the National Toxicology Program (NTP), increased incidences of squamous cell papillomas and carcinomas of the fore stomach were observed in rats and mice exposed via gavage (experimentally placing the chemical in the stomach). However, the NTP recently determined that these data were not relevant to human carcinogenicity since humans do not have a fore stomach, and removed ethyl acrylate from its list of carcinogens." (Occupational exposure generally involves exposure that occurs regularly, over an extended period of time.)One favorable safety aspect is that ethyl acrylate has good warning properties; the odor threshold is much lower than any level of health concern. In other words, the bad odor warns people of ethyl acrylate's presence long before the concentration reaches a level capable of creating a serious health risk.
Возможный контакт
This material is used in emulsion polymers for paints, textiles, adhesives, coatings and binders; as a monomer in the manufacture of homopolymer and copolymer resins for the production of paints and plastic filmsКанцерогенность
A retrospective study found an excess of colorectal cancers in one exposed population of workers; however, the data were confounded by other exposures and lack of association of causality and risk in similarly exposed populations from other locations. Therefore, there was inadequate evidence based on the study that ethyl acrylate is a human carcinogen . Ethyl acrylate is listed as USEPA group B2, “Probable human carcinogen”; IARC group B2, “Possibly carcinogenic in humans”; NIOSH, “Carcinogen with no further categorization”; NTP group 2, “Reasonably anticipated to be a carcinogen” and listed as a carcinogen by California Proposition 65 .Dermal studies of acrylic acid, ethyl acrylate, and n-butyl acrylate using mice did not result in local carcinogenesis, but several mice in the ethyl acrylate-treated group did exhibit dermatitis, dermal fibrosis, epidermal necrosis, and hyperkeratosis .
Экологическая судьба
Chemical/Physical. Polymerizes on standing and is catalyzed by heat, light, and peroxides (Windholz et al., 1983). Slowly hydrolyzes in water forming ethanol and acrylic acid. The reported rate constant for the reaction of ethyl acrylate with ozone in the gas phase was determined to be 5.70 x 10-18 cm3 mol/sec (Munshi et al., 1989).At an influent concentration of 1,015 mg/L, treatment with GAC resulted in an effluent concentration of 226 mg/L. The adsorbability of the carbon used was 157 mg/g carbon (Guisti et al., 1974).
Перевозки
UN1917 Ethyl acrylate, Hazard Class: 3; Labels: 3-Flammable liquidМетоды очистки
Wash the ester repeatedly with aqueous NaOH until free from inhibitors such as hydroquinone, then wash it with saturated aqueous CaCl2 and distil it under reduced pressure. Hydroquinone should be added if the ethyl acrylate is to be stored for extended periods. [Beilstein 2 IV 1460.] LACHRYMATORY.Несовместимости
May form explosive mixture with air. Atmospheric moisture and strong alkalies may cause fire and explosions unless properly inhibited (Note: Inert gas blanket not recommended). Heat, light or peroxides can cause polymerization. Incompatible with oxidizers (may be violent), peroxides, polymerizers, strong alkalis; moisture, chlorosulfonic acid, strong acids; amines. May accumulate static electrical charges, and may cause ignition of its vapors. Polymerizes readily unless an inhibitor, such as hydroquinone is added. Uninhibited vapors may plug vents by the formation of polymers.Утилизация отходов
Incineration or by absorption and landfill disposalЭТИЛАКРИЛАТ запасные части и сырье
сырьё
1of4
запасной предмет
- Ethyl 5-[3-(4,6-dimethoxy pyrimidyl-2-yl)ureido]methylpyrazolyl-4-carboxylic ester
- Этил 2-(бромметил)акрила
- Эналаприл
- Ethyl 3-(benzylamino)propanoate
- Ciprofloxacin hydrochloride
- Vinyl acetate-acrylate copolymer,emulsion
- Ethyl 3-(isopropylamino)propanoate
- Adhesive for electrostatic flocking
- carfentrazone-ethyl
- 2,4-пиперидиндион
- Этил 3 - (метилтио) пропионата
- acrylic resin coating finish
- Thickening agent
- Leather coating agent of filling acrylic resin
- impregnanting agent for leather
1of5
ЭТИЛАКРИЛАТ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8617732866630 | China | 8773 | 58 | |
| +8613288715578 | China | 12554 | 58 | |
| +86-13131129325 | China | 5251 | 58 | |
| +86-0532-86867058 +86-18562606086 |
China | 1066 | 58 | |
| +8613336195806 | China | 29733 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21622 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2988 | 55 | |
| +86 18953170293 | China | 2930 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 18631714998 | CHINA | 904 | 58 |
ЭТИЛАКРИЛАТ Обзор)
1of4