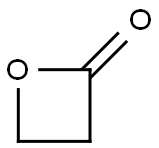
Бета-пропиолактон
- английское имя2-Oxetanone
- CAS №57-57-8
- CBNumberCB0275433
- ФормулаC3H4O2
- мольный вес72.06
- EINECS200-340-1
- номер MDLMFCD00005169
- файл Mol57-57-8.mol
| Температура плавления | -33 °C (lit.) |
| Температура кипения | 162 °C (lit.) |
| плотность | 1.146 g/mL at 25 °C (lit.) |
| давление пара | 3 at 25 °C (NIOSH, 1997) |
| показатель преломления | n |
| Fp | 158 °F |
| температура хранения | -20°C |
| растворимость | Miscible with acetone, alcohol, chloroform, and ether (Windholz et al., 1983) |
| форма | Liquid |
| цвет | Colorless liquid with a sweet but irritating odor |
| Запах | pungent odor |
| Растворимость в воде | 37 g/100 mL |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,7820 |
| констант закона Генри | 7.6 at 25 °C (approximate - calculated from water solubility and vapor pressure) |
| Стабильность | Moisture Sensitive, Very Hygroscopic |
| ИнЧИКей | VEZXCJBBBCKRPI-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 57-57-8(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | PROPIOLACTONE |
| FDA 21 CFR | 175.105 |
| Рейтинг продуктов питания EWG | 5 |
| FDA UNII | 6RC3ZT4HB0 |
| Предложение 65 Список | beta-Propiolactone |
| Справочник по химии NIST | «beta»-Propiolactone(57-57-8) |
| МАИР | 2B (Vol. 4, Sup 7, 71) 1999 |
| Система регистрации веществ EPA | beta-Propiolactone (57-57-8) |
| Коды опасности | T+ | |||||||||
| Заявления о рисках | 45-26-36/38 | |||||||||
| Заявления о безопасности | 53-45-99 | |||||||||
| РИДАДР | UN 3382 6.1/PG 1 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | RQ7350000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | I | |||||||||
| кода HS | 29322090 | |||||||||
| Банк данных об опасных веществах | 57-57-8(Hazardous Substances Data) | |||||||||
| Токсичность | LC50 (inhalation) for rats 25 ppm/6-h (quoted, RTECS, 1985). | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H350:Может вызывать раковые заболевания.
H330:Смертельно при вдыхании.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Бета-пропиолактон химические свойства, назначение, производство
Описание
Beta-propiolactone is a colorless liquid with a strong, slightly sweet odor. It may occur naturally, but no clear documentation of its occurrence in nature was found, and it must be synthesized for commercial purposes. Beta-propiolactone is unstable at room temperature but stable when stored at 5 ℃ in glass containers.Its tendency to be unstable and react with other molecules in the vicinity is responsible for both its toxicity and its usefulness. Significant commercial production of beta-propiolactone took place during the late 1950s through the mid-1970s, when it was widely used in chemical synthesis in reactions with other molecules to produce new chemicals. All lactones are characterized by a ring structure consisting of two or more carbon atoms – as can be seen from its structure, beta-propiolactone has three in its ring – and a single oxygen, coupled with an adjacent ketone. The fewer the carbons in the ring, the more ‘strained’ is the ring structure and the more unstable and reactive its characteristics. When the ring bonds break, the betapropiolactone molecules attach to other nearby molecules.
Химические свойства
β-Propiolactone is a colorless liquid which slowly hydrolyzes to hydracrylic acid and must be cooled to remain stable. It reacts with alcohols, acid chlorides, ammonia, and water to yield β-substituted propionic acid derivatives. The most important characteristic of the substance is its ability to polymerize. This highly exothermic process occurs simply by warming, although it is also catalyzed by both acid and base.Физические свойства
β-Propiolactone is a colorless, highly reactive liquid,thatis solublein water, alcohol, acetone, and chloroform (solubilityinwaterat 25℃, 37 vol %).Использование
Reacts with bacteriphage DNA causing inactivation, repair and recombinationОбщее описание
A colorless liquid with a slightly sweetish, pungent odor. Used as an intermediate in organic synthesis; disinfectant, sterilant for blood plasma, tissue grafts, vaccines, enzymes and surgical instruments.Реакции воздуха и воды
Slow reaction with water to form beta- hydroxypropionic acid.Профиль реактивности
2-Oxetanone is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. 2-Oxetanone may be incompatible with alkalis.Опасность
Strong skin and upper respiratory tract irri- tant, skin cancer. Possible carcinogen. Worker expo- sure should be minimized.Угроза здоровью
The toxicity potential of 2-Oxetanone via inhalation or ingestion is high; may cause death or permanent injury after very short exposures to small quantities. It is a carcinogen.Пожароопасность
Containers may explode. When heated to decomposition, 2-Oxetanone emits acrid smoke and fumes. Stable when stored at 41F. Avoid storing in areas of exposure to the direct rays of the sun and in areas of high fire hazard. Tends to polymerize on storage. Avoid elevated temperatures.Профиль безопасности
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Poison by inhalation. Moderately toxic by intraperitoneal route. An initiator. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
β-Propiolactone is used as a chemical intermediate in synthesis of acrylic acid and esters, acrylate plastics; as a vapor sterilizing agent; phase disinfectant; and a viricidal agent.Канцерогенность
β-Propiolactone is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.Экологическая судьба
Chemical/Physical. Slowly hydrolyzes to hydracrylic acid (Windholz et al., 1983). In a reactor heated to 250 °C and a pressure of 12 mmHg, β-propiolactone decomposed to give equal amounts of ethylene and carbon dioxide (James and Wellington, 1969).Перевозки
UN3382 Toxic by inhalation liquid, n.o.s. with an LC 50 ≤1000 mL/m 3 and saturated vapor concentration ≥10 LC 50 , Hazard class: 6.1; Labels: 6.1 Technical Name Required, Inhalation Hazard Zone B. UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.Методы очистки
Fractionally distil the lactone from sodium under reduced pressure. It gives an acidic solution in H2O. It irritates the skin and is a possibНесовместимости
Reacts with water, causing decomposi- tion and forming 3-hydroxypropionic acid (CAS: 503-66- 3), an irritant. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo- sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Incompatible with acet- ates, halogens, thiocyanates, thiosulfates, strong oxidizers; strong bases. Forms explosive mixture with air above 75℃. May polymerize upon storage or due to warming. Stable if kept under refrigeration @ 5 to 10 ℃/40 to 50 ℃.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.Бета-пропиолактон запасные части и сырье
сырьё
1of2
запасной предмет
- 3-меркаптопропионовая кислота
- 6-Bromo-2,3-dihydro-4H-chromen-4-one
- н-бутилакрилата
- dihydroxyethyl p-octadecyl phenylsulfonyl amino propyl ammoium propylsulfonate
- dimethyl p-octadecyl phenylsulfonyl amino propyl ammoium propylsulfonate
1of3
Бета-пропиолактон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +8615866703830 | China | 8497 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 |