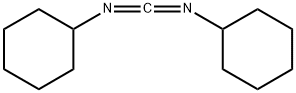
Дициклогексилкарбодиимид
- английское имяDicyclohexylcarbodiimide
- CAS №538-75-0
- CBNumberCB9706578
- ФормулаC13H22N2
- мольный вес206.33
- EINECS208-704-1
- номер MDLMFCD00011659
- файл Mol538-75-0.mol
| Температура плавления | 34-35 °C(lit.) |
| Температура кипения | 122-124 °C6 mm Hg(lit.) |
| Плотность накопления | 920kg/m3 |
| плотность | 1.247 g/mL at 25 °C |
| давление пара | 1.044-1.15Pa at 20-25℃ |
| показатель преломления | n |
| Fp | 190 °F |
| температура хранения | Store below +30°C. |
| растворимость | methylene chloride: 0.1 g/mL, clear, colorless |
| форма | Waxy Solid or Crystalline Mass |
| Удельный вес | 1.247 |
| цвет | White to pale yellow |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,3096 |
| БРН | 610662 |
| Стабильность | Stable, but moisture sensitive. Combustible. Incompatible with strong oxidizing agents. Avoid exposure to air or moisture. |
| ИнЧИКей | QOSSAOTZNIDXMA-UHFFFAOYSA-N |
| LogP | 5.567-6.83 at 25℃ |
| Справочник по базе данных CAS | 538-75-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1-3 |
| FDA UNII | 0T1427205E |
| Справочник по химии NIST | Methanediimine, n,n'-dicyclohexyl-(538-75-0) |
| Система регистрации веществ EPA | Dicyclohexylcarbodiimide (538-75-0) |
| UNSPSC Code | 12352200 |
| NACRES | NA.22 |
| Коды опасности | T,Xn,T+ | |||||||||
| Заявления о рисках | 23/24/25-34-40-43-41-36/38-21-24-22-62-37/38-10-61-26-38-20/22 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-41-24-37/39-24/25-36-16-53-28 | |||||||||
| РИДАДР | UN 2922 8/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | FF2160000 | |||||||||
| F | 3-8-10-21 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29252000 | |||||||||
| Банк данных об опасных веществах | 538-75-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 1110 mg/kg LD50 dermal Rat 71 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H318:При попадании в глаза вызывает необратимые последствия.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H336:Может вызывать сонливость или головокружение.
H312:Вредно при попадании на кожу.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Дициклогексилкарбодиимид химические свойства, назначение, производство
Описание
Dicydohexyl carbodiimide is used in peptide chemistry as a coupling reagent. It is both an irritant and a sensitizer, and caused contact dermatitis in pharmacists and chemists.Химические свойства
N,N0 -Dicyclohexylcarbodiimide (DCC) is a white crystalline solid. Odor is sweet and heavy.Использование
N,N'-Dicyclohexylcarbodiimide is a carbodiimide used to couple amino acids during peptide synthesis. N,N'-Dicyclohexylcarbodiimide is used as a dehydrating agent for the preparation of amides, ketones , nitriles as well as in the inversion and esterification of secondary alcohols.Определение
ChEBI: A carbodiimide compound having a cyclohexyl substituent on both nitrogen atoms.Подготовка
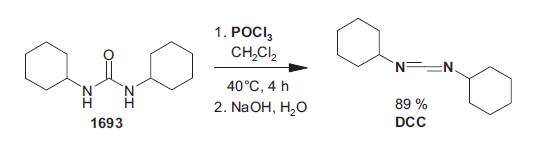
A stirred mixture of N,N′-dicyclohexylurea (19.7 g), phosphorus pentoxide (100 g), sand (175 g), and pyridine (700 mL) was refluxed for 2.25 h. Stirring was no longer possible after about 30 min. The mixture was filtered and the residue was extracted with pyridine (100 mL). Pyridine was removed from the combined solutions on a flash evaporator, and the residual oil was extracted with boiling petroleum ether (bp 60–80 C°) (2 × 100 mL), and then with diethyl ether (100 mL). The combined extracts were washed with iced water (3×80 mL), dried over calcium chloride, and filtered. The solvents were removed from the filtrate under reduced pressure to give 17.4 g of an oil, which on distillation yielded 13.7 g (76%) of a clear liquid; bp 143 C° (3.5 mmHg), which solidified in the receiver; mp 34–35 C°.Another method for producing DCC from dicyclohexylurea is a two-step process using phosphoryl chloride in dichloromethane at 40 C° for 4 h under non-basic conditions followed by removal of acidic components with aq. sodium hydroxide. This method, which gives an 89% yield of DCC, has been presented in a patent application.
Общее описание
White crystalline solid with a heavy sweet odor.Реакции воздуха и воды
Sensitive to moisture.Профиль реактивности
Dicyclohexylcarbodiimide is an imide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). Dicyclohexylcarbodiimide is incompatible with acids and oxidizing agents. Dicyclohexylcarbodiimide reacts with water.Опасность
A poison by skin contact. Moderately toxic by ingestion and inhalation.Пожароопасность
Dicyclohexylcarbodiimide is probably combustible.Контактные аллергены
Used in peptide chemistry as a coupling reagent. It is both an irritant and a sensitizer and has caused contact dermatitis in pharmacists and chemists.Возможный контакт
Laboratory reagentПеревозки
UN2928 Toxic solids, corrosive, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, 8- Corrosive material, Technical Name Required. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name RequiredМетоды очистки
It is sampled as a liquid after melting in warm H2O. It is sensitive to air, and it is a potent skin irritant. It can be distilled in a vacuum and is best stored in a tightly stoppered flask in a freezer. It is very soluble in CH2Cl2 and pyridine where the reaction product with H2O, after condensation, is dicyclohexyl urea which is insoluble and can be filtered off. Alternatively dissolve it in CH2Cl2, add powdered anhydrous MgSO4, shake for 4hours, filter, evaporate and distil at 0.6mm pressure and oil bath temperature of 145o. [Bodansky et al. Biochemical Preparations 10, 122 1963, Schmidt & Seefelder Justus Liebigs Ann Chem 571 83 1951, Schmidt et al. Justus Liebigs Ann Chem 612 11 1958, Beilstein 12 IV 72.]Несовместимости
Dust may for explosive mixture with air. Reacts with steam and water. N,N0 - Dicyclohexylcarbodiimide is an amine/imide: contact with strong oxidizers may cause fire and explosions. Incompatible with acids, strong bases, strong reducing agents (may form flammable gasses); azo and diazo compounds (may form toxic gases); chlorinated hydrocarbons; nitro compounds. Contact with mixture of acetic acid 1 dinitrogen trioxide may cause explosion. The combustion of amide compounds generate nitrogen oxides (NOx). In the presence of moisture, may attack metals and plastics.Утилизация отходов
Whatever cannot be saved for recovery or recycling should be managed in an appropriate and approved waste facility. Although not a listed RCRA hazardous waste, this material may exhibit one or more characteristics of a hazardous waste and require appropriate analysis to determine specific disposal requirements. Processing, use or contamination of this product may change the waste management options. State and local disposal regulations may differ from federal disposal regulations. Dispose of container and unused contents in accordance with federal, state and local requirementsДициклогексилкарбодиимид запасные части и сырье
сырьё
1of2
запасной предмет
- Латанопрост
- Эналаприл
- Бета-аланин-трет-бутилэфир гидрохлорид
- Octreotide acetate
- 7-DIETHYLAMINO-3-THENOYLCOUMARIN
- 2,6-ДИХЛОРФЕНЕТИЛИЗОЦИАНИД
- ТРЕТ-БУТИЛ 3-ГИДРОКСИПРОПИОНАТ
- 2-морфолиноизоникотинальдегид
- Cefprozil hydrate
- Lisinopril Dihydrate
- ГЛИ-7-АМИНО-4-МЕТИЛКУМАРИН
- 4-ISOCYANOBENZOPHENONE
- Пароксетин
- Каптоприл
- 2-CYANOPYRIDINE-4-CARBOXALDEHYDE
- Цефодизим
- 1-(2-ИЗОЦИАНОЭТИЛ)-4-МЕТИЛПИПЕРАЗИН
- (S)-(-)-3-Аминохинуклидин дигидрохлорид
- Цефтазидим
- 3-CARBETHOXY-4-HYDROXYCOUMARIN
1of7
Дициклогексилкарбодиимид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13806087780 | China | 17365 | 58 | ||
| +8613401983379 | CHINA | 74 | 58 | ||
| +86-18553607090 +8618553607090 |
China | 1667 | 58 | ||
| +undefined18621330623 | China | 28 | 58 | ||
| +86-18958038633; +8618958038633 |
China | 64 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-86-13583358881 +8618560316533 |
China | 3094 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 |