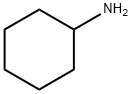
ЦИКЛОГЕКСИЛАМИН
- английское имяCyclohexylamine
- CAS №108-91-8
- CBNumberCB8139274
- ФормулаC6H13N
- мольный вес99.17
- EINECS203-629-0
- номер MDLMFCD00001486
- файл Mol108-91-8.mol
| Температура плавления | -17 °C |
| Температура кипения | 134 °C(lit.) |
| плотность | 0.867 g/mL at 25 °C(lit.) |
| плотность пара | 3.42 (vs air) |
| давление пара | 10 mm Hg ( 22 °C) |
| показатель преломления | n |
| Fp | 90 °F |
| температура хранения | Store below +30°C. |
| растворимость | organic solvents: miscible |
| пка | 10.66(at 24℃) |
| форма | Liquid |
| цвет | Clear |
| РН | 11.5 (100g/l, H2O, 20℃) |
| Запах | strong fishy odor |
| Пределы взрываемости | 1.6-9.4%(V) |
| Растворимость в воде | MISCIBLE |
| Точка замерзания | -17.7℃ |
| Чувствительный | Air Sensitive |
| Мерк | 14,2729 |
| БРН | 471175 |
| Пределы воздействия | TLV-TWA 10 ppm (~40 mg/m3) (ACGIH). |
| Диэлектрическая постоянная | 5.3(-21℃) |
| ИнЧИКей | PAFZNILMFXTMIY-UHFFFAOYSA-N |
| LogP | 3.7 at 25℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | CYCLOHEXYLAMINE |
| Справочник по базе данных CAS | 108-91-8(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2-5 |
| FDA UNII | I6GH4W7AEG |
| Справочник по химии NIST | Cyclohexanamine(108-91-8) |
| Система регистрации веществ EPA | Cyclohexylamine (108-91-8) |
| UNSPSC Code | 12352116 |
| NACRES | NA.22 |
| Коды опасности | C,T | |||||||||
| Заявления о рисках | 10-21/22-34-62-24-22 | |||||||||
| Заявления о безопасности | 36/37/39-45-1/2-26 | |||||||||
| РИДАДР | UN 2357 8/PG 2 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 10 ppm (40 mg/m3) | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | GX0700000 | |||||||||
| F | 10-34 | |||||||||
| Температура самовоспламенения | 559 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2921 30 10 | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 108-91-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 0.71 ml/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H301+H311:Токсично при проглатывании или при контакте с кожей.
H302+H312:Вредно при проглатывании или при попадании на кожу.
H361fd:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению. Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
ЦИКЛОГЕКСИЛАМИН химические свойства, назначение, производство
Химические свойства
Cyclohexylamine is a colorless to yellow liquid (amines, primary aromatic). It has an unpleasant fishy odor. Flammable. It is infinitely miscible with water and conventional organic solvents. With water it forms an azeotrope that contains 44.2 % cyclohexylamine and boils at 96.4℃. Cyclohexylamine can be volatilized with water vapor. It can absorb carbon dioxide in the air and form a white crystalline carbonate. Aqueous solution is alkaline. 0.01% concentration of aqueous solution pH = 10.5. Its vapor and air to form an explosive mixture.Использование
Cyclohexylamine is used in the manufacture of a number of products, including plasticizers, drycleaning soaps, insecticides, and emulsifying agents. It is also used as a corrosion inhibitor and in organic synthesis.Определение
ChEBI: Cyclohexylamine is a primary aliphatic amine consisting of cyclohexane carrying an amino substituent. It has a role as a human xenobiotic metabolite and a mouse metabolite. It is a conjugate base of a cyclohexylammonium.Методы производства
Cyclohexylamine is produced by the reaction of ammonia and cyclohexanol at elevated temperature and pressure in the presence of a silica-alumina catalyst (SRI 1985). It is also prepared by a similar process of catalytic hydrogenation of aniline at elevated temperature and pressure. Fractionation of the product of this reaction yields CHA, aniline, and a high-boiling residue containing n-phenylcyclohexylamine and dicyclohexylamine (Carswell and Morrill 1937). In 1982, U.S. production was 4.54 metric tons and 739.3 metric tons were imported into the U.S. (SRI 1985).Общее описание
Cyclohexylamine appears as a clear colorless to yellow liquid with an odor of ammonia. Flash point 90 °F. Irritates the eyes and respiratory system. Skin contact may cause burns. Less dense than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion.Реакции воздуха и воды
Highly flammable. Sensitive to air and light. Soluble in water.Профиль реактивности
Cyclohexylamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.Угроза здоровью
Cyclohexylamine is a severe irritant to theeyes, skin, and respiratory passage. Skincontact can produce burns and sensitization;contact of the pure liquid or its concentratedsolutions with the eyes may cause loss ofvision.The acute oral and dermal toxicity ofcyclohexylamine was moderate in test sub jects. The toxic effects include nausea, vom iting, and degenerative changes in the brain,liver, and kidney. Inhalation of its vaporsat high concentrations may cause a narcoticeffect.
LD50 value, oral (rats): 156 mg/kg
LD50 value, skin (rabbits): 277 mg/klg
Cyclohexylamine may be mutagenic, thetest for which has so far given inconclusiveresults. Administration of this compoundin animals produced a reproductive effect,including embryotoxicity and a reductionin male fertility. Intraperitoneal injectionof the amine in rats caused a dose dependent increase in chromosomal breaks.Roberts and coworkers (1989) studied themetabolism and testicular toxicity of cyclohexylamine (a metabolite of cyclamate)in rats and mice. Chronic dietary administration of 400 mg/kg/day for 13 weeksshowed decrease in organ weigh, histological changes, and testicular atrophy in boththe Wistar and dark agouti DA rats, but to awidely varying extent, while mice exhibitedno evidence of testicular damage.
There is no evidence of carcinogenicityin animals or humans caused by cyclohexy lamine.
Пожароопасность
When heated to decomposition, Cyclohexylamine emits highly toxic fumes. Vapor may travel a considerable distance to source of ignition and flash back. Toxic oxides of nitrogen are produced during combustion. Nitric acid; reacts vigorously with oxiding materials. Stable, avoid physical damage, storage with oxidizing material.Промышленное использование
The primary use of cyclohexylamine is as a corrosion inhibitor in boiler water treatment and in oil field applications (HSDB 1989). It is also a chemical intermediate for rubber processing chemicals, dyes (acid blue 62, former use), cyclamate artificial sweeteners and herbicides and a processing agent for nylon fiber production (SRI 1985). Windholz et al (1983) reports its use in the manufacture of insecticides, plasticizers, emulsifying agents, dry-cleaning soaps, and acid gas absorbents.Профиль безопасности
A poison by ingestion, skin contact, and intraperitoneal routes. Experimental teratogenic and reproductive effects. A severe human skin irritant. Can cause dermatitis and convulsions. Human mutation data reported. Questionable carcinogen. Flammable liquid. Dangerous fire hazard when exposed to heat, flame, or oxidizers. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx.Синтез
Prepared by catalytic hydrogenation of aniline at elevated temp and pressures. Fractionation of crude reaction product yields cyclohexylamine, unchanged aniline, and high-boiling residue containing n-phenylcyclohexylamine (cyclohexylaniline) and dicyclohexylamine.Возможный контакт
CHA is used in making dyes, chemi- cals, dry cleaning chemicals; insecticides, plasticizers, rub- ber chemicals; and as a chemical intermediate in the production of cyclamate sweeteners. Used in water treat- ment and as a boiler feedwater additive. It is also used in rubber production to retard degradation.Канцерогенность
According to the International Agency for Research of Cancer (IARC) working group, there is no evidence that cyclohexylamine is teratogenic or carcinogenic.хранилище
Cyclohexylamine can be stored and shipped in iron tanks. Nonferrous metals, particularly copper-containing materials, are attacked and are therefore unsuitable. The amine discolors on contact with air and therefore must be kept under nitrogen.Перевозки
UN2357 Cyclohexylamine, Hazard class: 8; Labels: 8-Corrosive material, 3-Flammable liquid.Методы очистки
Dry the amine with CaCl2 or LiAlH4, then distil it from BaO, KOH or Na, under N2. Also purify it by conversion to the hydrochloride (which is crystallised several times from water), then liberation of the amine with alkali and fractional distillation under N2. The hydrochloride has m 205-207o (dioxane/EtOH). [Lycan et al. Org Synth Coll Vol II 319 1943, Beilstein 12 III 10, 12 IV 8.]Несовместимости
May form explosive mixture with air. Cyclohexylamine is a strong base: it reacts violently with acid. Contact with strong oxidizers may cause fire and explosion hazard. Incompatible with organic anhydrides; isocyanates, vinyl acetate; acrylates, substituted allyls; alkylene oxides; epichlorohydrin, ketones, aldehydes, alco- hols, glycols, phenols, cresols, caprolactum solution; lead. Corrosive to copper alloys, zinc, or galvanized steel.Утилизация отходов
Incineration; incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissions.ЦИКЛОГЕКСИЛАМИН запасные части и сырье
ЦИКЛОГЕКСИЛАМИН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13806087780 | China | 17365 | 58 | ||
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | ||
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | ||
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | ||
| China | 5701 | 58 | |||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 |