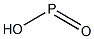
Hypophosphorous acid
- английское имяHypophosphorous acid
- CAS №6303-21-5
- CBNumberCB6130318
- ФормулаHO2P
- мольный вес63.980501
- EINECS228-601-5
- номер MDLMFCD02183592
- файл Mol6303-21-5.mol
| Температура плавления | -25 °C |
| Температура кипения | 108 °C (759.8513 mmHg) |
| плотность | 1.206 g/mL at 20 °C(lit.) |
| давление пара | <17 mmHg ( 20 °C) |
| температура хранения | no restrictions. |
| растворимость | very soluble in H2O, ethanol, ethyl ether |
| пка | pK1 1.1. |
| форма | hygroscopic crystals or colorless oily liquid |
| цвет | Colorless |
| РН | pKa1= 1.00(25℃) |
| Растворимость в воде | SOLUBLE |
| Мерк | 13,4894 |
| Стабильность | Stable. Incompatible with strong bases. Reacts violently with oxidizing agents, strong bases, mercury (II) nitrate and mercury (II) oxide. Do not heat above 100 C. |
| ИнЧИКей | GQZXNSPRSGFJLY-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 6303-21-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 8B1RL9B4ZJ |
| Справочник по химии NIST | Hypophosphorous acid(6303-21-5) |
| Система регистрации веществ EPA | Phosphinic acid (6303-21-5) |
| UNSPSC Code | 12352301 |
| NACRES | NA.21 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 34 | |||||||||
| Заявления о безопасности | 26-36/37/39-45 | |||||||||
| РИДАДР | UN 3264 8/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | SZ6400000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28111990 | |||||||||
| Банк данных об опасных веществах | 6303-21-5(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H290:Может вызывать коррозию металлов.
-
оператор предупредительных мер
P234:Хранить только в оригинальной упаковке.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Hypophosphorous acid химические свойства, назначение, производство
Описание
Hypophosphorous acid is a powerful reducing agent with a molecular formula of H3PO2. Inorganic chemists refer to the free acid by this name although its IUPAC name is dihydridohydroxidooxidophosphorus, or the acceptable name of phosphinic acid. It is a colorless low-melting compound, which is soluble in water, dioxane, and alcohols. The formula for hypophosphorous acid is generally written H3PO2, but a more descriptive presentation is HOP(O)H2 which highlights its monoprotic character. Salts derived from this acid are called phosphinates (hypophosphites).Химические свойства
colourless liquidФизические свойства
Colorless deliquescent crystals or oily liquid; sour odor; density 1.493 g/cm3;melts at 26.5°C; boils at 130°C; very soluble in water, alcohol and ether; den-sity of a 50% aqueous solution is 1.13 g/mL.Использование
Hypophosphorous acid is primarily used for electroless nickel plating. It is involved in the reduction of arenediazonium salts. It acts as an additive in Fischer esterification reactions. Also, it serves as a neutralizing agent, antioxidant, catalyst in polymerization and poly condensation, and wetting agent. Further, it is used in the formulation of pharmaceuticals, discoloration of polymers, water treatment and retrieval of precious or non-ferrous metals. In addition to this, it is used as bleaching agents for plastics, synthetic fibers, decolorizing agent and for color stabilization during the manufacture of chemicals and several plastics.Методы производства
Hypophosphorous acid is formed by reaction of barium hypophosphite and sulfuric acid, and filtering off barium sulfate. By evaporation of the solution in vacuum at 80 °C, and then cooling to 0°C, hypophosphorous acid crystallizes.Определение
ChEBI: A phosphorus oxoacid that consists of a single pentavalent phosphorus covalently bound via single bonds to two hydrogens and a hydroxy group and via a double bond to an oxygen. The parent of the class of phosphinic acids.Подготовка
Hypophosphorous acid may be prepared by various methods:1. Boiling white phosphorus with calcium hydroxide:
P4 + 4Ca(OH)2 + 8H2O → 4Ca(H2PO2)2 + 4H2
The calcium salt is soluble in water. Treatment with sulfuric acid yields thehypophosphorous acid:
(H2PO2)2Ca + H2SO4 → 2H3PO2 + CaSO4
The product mixture is filtered to remove insoluble CaSO4. The aqueous solu-tion of hypophosphorous acid is concentrated under reduced pressure.Concentrated baryta water may be used instead of calcium hydroxide.2. By treating sodium hypophosphite, NaH2PO2with an ion-exchange resin.The sodium salt may be produced by boiling white phosphorus with a solutionof sodium hydroxide, a reaction similar to (1) above.
PH3 + 2I2 + 2H2O → H3PO2 + 4HI
The above method may be considered safer than that involving heating whitephosphorus with an alkali.
Hypophosphorous acid must be stored below 50°C. It is sold commerciallyas an aqueous solution at various concentrations.
Реакции
Hypophosphorous acid is miscible with water in all proportions and a commercial strength is 30% H3PO2. Hypophosphites are used in medicine. Hypophosphorous acid is a powerful reducing agent, e.g., with copper sulfate forms cuprous hydride Cu2H2, brown precipitate, which evolves hydrogen gas and leaves copper on warming; with silver nitrate yields finely divided silver; with sulfurous acid yields sulfur and some hydrogen sulfide; with sulfuric acid yields sulfurous acid, which reacts as above; forms manganous immediately with permanganate.Общее описание
Hypophosphorous acid appears as colorless oily liquid or deliquescent crystals with a sour odor. Density 1.439 g / cm3. Melting point 26.5°C. Inhalation of vapors irritates or burns the respiratory tract. Liquid and vapors may irritate or burn eyes and skin.Реакции воздуха и воды
Deliquescent. Water soluble.Профиль реактивности
HYPOPHOSPHOROUS ACID decomposes when heated into phosphoric acid and spontaneously flammable phosphine. Is oxidized by sulfuric acid with release of sulfur dioxide and sulfur. Reacts explosively with mercury(II) oxide [Mellor, 1940, Vol. 4, 778]. Reacts violently with mercury(II) nitrate [Mellor, 1940, Vol. 4, 993]. Neutralizes bases in exothermic reactions.Опасность
Fire and explosion risk in contact with oxidizing agents.Угроза здоровью
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Методы очистки
Phosphorous acid is a common contaminant of commercial 50% hypophosphorous acid. Jenkins and Jones [J Am Chem Soc 74 1353 1952] purified this material by evaporating about 600mL in a 1L flask at 40o, under reduced pressure (in N2), to a volume of about 300mL. After the solution was cooled, it was transferred to a wide-mouthed Erlenmeyer flask which was stoppered and left in a Dry-ice/acetone bath for several hours to freeze (if necessary, with scratching of the wall). When the flask was then left at ca 5o for 12hours, about 30-40% of it liquefied, and was again filtered. This process was repeated, then the solid was stored over Mg(ClO4)2 in a vacuum desiccator in the cold. Subsequent crystallisations from n-butanol by dissolving it at room temperature and then cooling in an ice-salt bath at -20o did not appear to purify it further. The free acid forms deliquescent crystals m 26.5o and is soluble in H2O and EtOH. The NaH2PO2 salt can be purified through an anion exchange resin [Klement Z Anorg Allgem Chem 260 267 1949.]Hypophosphorous acid запасные части и сырье
Hypophosphorous acid поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8613223293093 | China | 80 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| 0086-571-86990109 | China | 104 | 55 | ||
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 | ||
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 |
Hypophosphorous acid Обзор)
- Дифенилфосфоrил азид
- Фенилфосфоновая кислота
- Диэтилцианометилфосфонатом
- Phenylphosphinic кислота
- Метилфосфоновой кислоты
- Метилендифосфоновой кислота
- Фенил дихлорфосфа
- Дифенилфосфиновая кислота
- ТРИС(2-ХЛОРЭТИЛ) ФОСФАТ
- Диэтиловый 2-bromoethylphosphonate
1of4