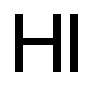
КИСЛОТА йодистоводородная
- английское имяHydriodic acid
- CAS №10034-85-2
- CBNumberCB7852570
- ФормулаHI
- мольный вес127.91
- EINECS233-109-9
- номер MDLMFCD00011347
- файл Mol10034-85-2.mol
| Температура плавления | -50.8° |
| Температура кипения | 127 °C(lit.) |
| плотность | 1.96 g/mL at 20 °C |
| давление пара | 721.8kPa at 20℃ |
| Fp | 126-127°C |
| температура хранения | 2-8°C |
| растворимость | very soluble in H2O; soluble in organic solvents |
| пка | -10(at 25℃) |
| форма | colorless or yellow gas |
| цвет | Colorless to brown |
| Запах | Pungent odor |
| Водородный показатель | 1 |
| РН | 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution) |
| Растворимость в воде | soluble |
| Чувствительный | Hygroscopic |
| Мерк | 14,4776 |
| Пределы воздействия | ACGIH: TWA 0.01 ppm |
| Диэлектрическая постоянная | 3.4(-50℃) |
| Стабильность | Stable. Incompatible with bases, amines. Corrodes steel. May discolour on exposure to air and light. |
| Справочник по базе данных CAS | 10034-85-2(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | HYDRIODIC ACID |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 694C0EFT9Q |
| Справочник по химии NIST | Hydrogen iodide(10034-85-2) |
| Система регистрации веществ EPA | Hydriodic acid (10034-85-2) |
| UNSPSC Code | 12352301 |
| NACRES | NA.23 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 34-35 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-9 | |||||||||
| РИДАДР | UN 1787 8/PG 2 | |||||||||
| WGK Германия | - | |||||||||
| RTECS | MW3760000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28111990 | |||||||||
| Банк данных об опасных веществах | 10034-85-2(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H411:Токсично для водных организмов с долгосрочными последствиями.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H290:Может вызывать коррозию металлов.
-
оператор предупредительных мер
P234:Хранить только в оригинальной упаковке.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
КИСЛОТА йодистоводородная химические свойства, назначение, производство
Описание
‘Iodine’ is derived from iodes, a Greek word meaning violet. It is a member of the halide family and hydrogen iodide is considered a strong acid.Химические свойства
Hydrogen iodide is a colourless to yellow/brown with an acrid odour non-flammable gas. Hydrogen iodide is incompatible with water and other halides. Hydrogen iodide, upon contact with moisture in air, releases dense vapours. Hydrogen iodide reacts with water to form corrosive acids and reacts violently with alkalis. Most metals corrode rapidly on contact with wet hydrogen iodide, and prolonged exposure of hydrogen iodide to fire or intense heat has been reported to cause the container to rupture and rocket.Физические свойства
This is a strong acid, made by dissolving HI gas in water. However, hydrogen iodide and hydroiodic acid differ in that the former is a gas under standard conditions whereas the other is an aqueous solution of said gas. They are noninterconvertible. That is, once the acid is formed with water, it cannot be recovered like HCl or HBr. Hydroiodic acid is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.With moist air, HI gas gives a mist (or fumes) of hydroiodic acid. It is exceptionally soluble in water. One liter of water will dissolve 425 L of HI, the final solution containing only four water molecules per molecule of HI. As stated, although chemically related, hydroiodic acid is not pure HI but a mixture containing it. Commercial “concentrated” hydroiodic acid usually contains 90–98% HI by mass.
Использование
Hydriodic acid is used in the manufactureof iodides, as a reducing agent, and indisinfectants and pharmaceuticals.Определение
hydrogen iodide: A colourless gas,HI; m.p. –51°C; b.p. –35.38°C. It canbe made by direct combination ofthe elements using a platinum catalyst.It is a strong acid dissociating extensivelyin solution (hydroiodic acid or hydriodic acid). It is also a reducingagent.Подготовка
Hydrogen iodide is prepared by direct combination of hydrogen and iodinevapor in the presence of platinum catalyst:H2 + I2 → 2HI
The compound is produced in commercial scale by reaction of iodine withhydrazine or hydrogen sulfide:
2I2 + N2H4 → 4HI + N2
I2 + H2S → 2HI + S
Hydriodic acid may be prepared by dissolving hydrogen iodide gas in water.The acid also may be obtained by electrolysis of iodine solution or by passinghydrogen sulfide into a suspension of iodine in water and boiling to expelexcess sulfide. After boiling, the precipitated sulfur is removed by filtrationthrough fritted glass plate or glass wool.
Hydriodic acid in small quantities may be prepared by adding water care-fully to a solid mixture of red phosphorus and iodine.
Technical grade hydriodic acid is a 47% HI solution and usually has abrown color due to the presence of free iodine, produced by air oxidation of HI.Hydriodic acid should be stored in the dark to prevent photochemical decom-position, and free from air to prevent oxidation. The addition of 1.5%hypophosphorus acid (H3PO2) prevents oxidative decomposition.
Hydriodic acid also is commercially sold at 57% (azeotropic concentration)and 10% aqueous solutions.
Общее описание
A colorless to yellow liquid with a pungent odor. Consists of a solution of hydrogen iodide in water. Fumes irritate the eyes and mucous membranes. Corrosive to metals and to tissue.It is prepared by the reaction of iodine and hydrosulfuric acid or by the reaction of phosphorus plus iodine plus water followed by distillation. Concentrated hydroiodic acid reacts with the oxygen of the air to form free iodine, which gives a brownish color to the solution. It also gives an idea of the reducing nature of this acid. It is an important reagent in organic chemistry and is used commercially in the preparation of iodides.
Реакции воздуха и воды
Soluble in water with release of heat.Профиль реактивности
HYDROIODIC ACID reacts exothermically with organic bases (amines, amides) and inorganic bases (oxides and hydroxides of metals). Reacts exothermically with carbonates (including limestone and building materials containing limestone) and hydrogen carbonates to generate carbon dioxide. Reacts with sulfides, carbides, borides, and phosphides to generate toxic or flammable gases. Reacts with many metals (including aluminum, zinc, calcium, magnesium, iron, tin and all of the alkali metals) to generate flammable hydrogen gas. Reacts violently with acetic anhydride, 2-aminoethanol, ammonium hydroxide, calcium phosphide, chlorosulfonic acid, 1,1-difluoroethylene, ethylenediamine, ethyleneimine, oleum, perchloric acid, b-propiolactone, propylene oxide, silver perchlorate/carbon tetrachloride mixture, sodium hydroxide, uranium(IV) phosphide, vinyl acetate, calcium carbide, rubidium carbide, cesium acetylide, rubidium acetylide, magnesium boride, mercury(II) sulfate [Lewis]. Mixtures with concentrated sulfuric acid can evolve toxic hydrogen iodide gas at a dangerous rate. Decomposes at high temperatures to emit toxic products. Reacts with fluorine, dinitrogen trioxide, nitrogen dioxide/dinitrogen tetraoxide, and fuming nitric acid.Опасность
Strong irritant. Poison.Угроза здоровью
Hydriodic acid is a corrosive liquid thatcan produce burns on contact with the skin.Contact of acid with the eyes can causesevere irritation. The gas, hydrogen iodide, isa strong irritant to the eyes, skin, and mucousmembranes. No exposure limit has been setfor this gas.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Методы очистки
Iodine can be removed from aqueous HI, probably as the amine hydrogen triiodide, by three successive extractions using a 4% solution of Amberlite LA-2 (a long-chain aliphatic amine) in CCl4, toluene or pet ether (10mL per 100mL of acid). [Davidson & Jameson Chem Ind (London) 1686 1963.] Extraction with tributyl phosphate in CHCl3 or other organic solvents is also suitable. Alternatively, a De-acidite FF anion-exchange resin column in the OH--form using 2M NaOH, then into its I--form by passing dilute KI solution through, can be used. Passage of an HI solution under CO2 through such a column removes polyiodide. The column can be regenerated with NaOH. [Irving & Wilson Chem Ind (London) 653 1964]. The earlier method was to reflux with red phosphorus and distil in a stream of N2. The colourless product is stored in ampoules in the dark [Bradbury J Am Chem Soc 74 2709 1952, Heisig & Frykholm Inorg Synth I 157 1939]. It fumes in moist air. HARMFUL VAPOURS.КИСЛОТА йодистоводородная запасные части и сырье
КИСЛОТА йодистоводородная поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +1-+1(833)-552-7181 | United States | 57505 | 58 | ||
| +8615387054039 | China | 427 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 | ||
| +8619521488211 | China | 2558 | 58 | ||
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | ||
| +8618523575427 | China | 49732 | 58 | ||
| 18503026267 | CHINA | 9636 | 58 | ||
| 86-512-58389969 | CHINA | 111 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8618633865755 | CHINA | 955 | 58 |
КИСЛОТА йодистоводородная Обзор)
1of4