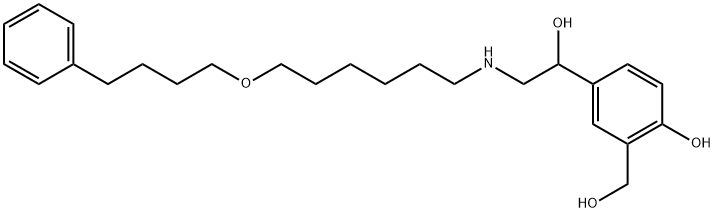
Салметерол
- английское имяSalmeterol
- CAS №89365-50-4
- CBNumberCB9186866
- ФормулаC25H37NO4
- мольный вес415.57
- номер MDLMFCD00867037
- файл Mol89365-50-4.mol
химическое свойство
| Температура плавления | 75.5-76.5° |
| Температура кипения | 603.0±55.0 °C(Predicted) |
| плотность | 1.112±0.06 g/cm3(Predicted) |
| температура хранения | 2-8°C |
| растворимость | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| пка | 9.99±0.31(Predicted) |
| форма | Solid |
| цвет | White to Off-White |
| Справочник по базе данных CAS | 89365-50-4(CAS DataBase Reference) |
| FDA UNII | 2I4BC502BT |
| Код УВД | R03AC12 |
| RTECS | CZ6457000 |
| Банк данных об опасных веществах | 89365-50-4(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Салметерол химические свойства, назначение, производство
Использование
Salmeterol is a β2-Adrenergic agonist used for relief and control of asthma symptoms.Определение
ChEBI: A phenol having a hydroxymethyl group at C-2 and a 1-hydroxy-2-{[6-(4-phenylbutoxy)hexyl]amino}ethyl group at C-4; derivative of phenylethanolamine.Общее описание
Salmeterol has an N-phenylbutoxyhexyl substituent incombination with a β-OH group and a salicyl phenyl ring foroptimal direct-acting β2-receptor selectivity and potency. Ithas a potency similar to that of ISO. This drug associateswith the β2-receptor slowly resulting in slow onset of actionand dissociates from the receptor at an even slower rate. It is resistant to both MAO and COMT and highly lipophilic(log P=3.88). It is thus very long acting (12 hours), aneffect also attributed to the highly lipophilic phenylalkylsubstituent on the nitrogen atom, which is believed to interactwith a site outside but adjacent to the active site.
Опасность
A poison by inhalation.Биологическая активность
Potent and selective β 2 -adrenoceptor agonist (EC 50 = 5.3 nM); bronchodilator. Unlike other β 2 agonists, binds to exo-site domain of β 2 receptors, producing a slow onset of action and prolonged activation. Also available as part of the β -Adrenoceptor Agonist Tocriset™ .Механизм действия
A β2-agonist with a slow onset and extended duration of action is salmeterol. Salmeterol has the same phenyl ring substitution R3 as albuterol but also an unusually long and lipophilic group R1 on the nitrogen. The octanol–water partition coefficient, log P, for salmeterol is 3.88, compared with 0.66 for albuterol. Salmeterol is approximately 50-fold more selective than albuterol for the β2-receptor. Substantial evidence indicates that its long duration of action results from a specific binding interaction (“anchoring”) of the phenyl group at the end of the extended lipophilic side chain with a specific region of the β2-receptor, affording salmeterol a unique binding mechanism.Клиническое использование
Salmeterol usually is prescribed for severe persistent asthma following previous treatment with a short-acting β-agonist, such as albuterol. The noticeable differences between salmeterol and albuterol are the onset of action and their duration of action (Table 13.6). When used regularly every day as prescribed, inhaled albuterol decreases the number and severity of asthma attacks. It is not used, however, for relieving an asthma attack that has already started.Побочные эффекты
Hoarseness, throat irritation, headache, rapid heartbeat, nervousness, cough, dry mouth/throat, or upset stomach may occur. To relieve dry mouth, suck on (sugarless) hard candy or ice chips, chew (sugarless) gum, drink water, or use a saliva substitute. This medication may raise your blood pressure. Rarely, this medication may cause sudden breathing problems/asthma right after you use it. If this occurs, use your quick-relief inhaler and get medical help right away.Метаболизм
Salmeterol is metabolized predominantly through CYP3A4, an isoform of cytochrome P450. CYP3A4 is responsible for the aliphatic oxidation of the salmeterol base. Salmeterol is extensively metabolized by hydroxylation into alpha-hydroxy-salmeterol and subsequently eliminated through feces and urine. Salmeterol is 57.4% eliminated in the feces and 23% in the urine. At recommended doses, systemic concentrations of salmeterol are low or undetectable. Only at very high doses are blood concentrations increased.Салметерол запасные части и сырье
Салметерол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 01056380788-8515; +8618518759099 |
China | 52 | 58 | ||
| +86-0533-2185556 +8617865335152 |
China | 10986 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7738 | 55 | ||
| 15950718863 | CHINA | 295 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 18627774460 | CHINA | 742 | 58 | ||
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | ||
| 18503026267 | CHINA | 9636 | 58 | ||
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 |