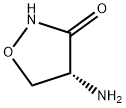
Д-циклосерин
- английское имяD-Cycloserine
- CAS №68-41-7
- CBNumberCB9138759
- ФормулаC3H6N2O2
- мольный вес102.09
- EINECS200-688-4
- номер MDLMFCD00005353
- файл Mol68-41-7.mol
химическое свойство
| Температура плавления | 147 °C (dec.)(lit.) |
| альфа | 111 º (C=5, 2N NaOH) |
| Температура кипения | 191.38°C (rough estimate) |
| плотность | 1.3516 (rough estimate) |
| показатель преломления | 1.5110 (estimate) |
| температура хранения | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| растворимость | water: soluble50mg/mL, clear, colorless to light yellow |
| форма | powder |
| пка | pKa 4.5 (Uncertain) |
| цвет | white to off-white |
| оптическая активность | [α]20/D +115.0±5.0°, c = 2% in H2O |
| Биологические источники | synthetic |
| Растворимость в воде | SOLUBLE |
| Чувствительный | Air Sensitive |
| Мерк | 14,2751 |
| БРН | 80798 |
| BCS Class | 3/1 |
| Стабильность | Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20°C for up to 1 month. |
| Справочник по базе данных CAS | 68-41-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| Словарь онкологических терминов NCI | D-cycloserine; Seromycin |
| FDA UNII | 95IK5KI84Z |
| Словарь наркотиков NCI | D-cycloserine |
| Код УВД | J04AB01 |
| Справочник по химии NIST | Cycloserine(68-41-7) |
| UNSPSC Code | 12352209 |
| NACRES | NA.28 |
больше
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 5-20 | |||||||||
| Заявления о безопасности | 38-36/37-24/25 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | NY2975000 | |||||||||
| F | 10-23 | |||||||||
| кода HS | 29419090 | |||||||||
| Банк данных об опасных веществах | 68-41-7(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
Д-циклосерин химические свойства, назначение, производство
Химические свойства
White to pale yellow cryst. powderИспользование
D-Cycloserine inhibits cell wall biosynthesis (D-Ala peptide bond formation). D-Cycloserine also prevents conversion of D-Ala to L-Ala. D-Cycloserine is an bacteriostatic. D-Cycloserine is an antibiot ic against Gram-negative bacteria.Показания
Cycloserine is a broad-spectrum antibiotic produced by Streptomyces orchidaceus. It is structural analogue of Dalanine and acts through a competitive inhibition of the D-alanine that is involved in bacterial cell wall synthesis. Cycloserine is inhibitory to M. tuberculosis and active against Escherichia coli, S. aureus, and Enterococcus, Nocardia, and Chlamydia spp. It is used in the treatment of MDR tuberculosis and is useful in renal tuberculosis, since most of the drug is excreted unchanged in the urine.Общее описание
Chemical structure: amino acid derivativesМеханизм действия
D-Cycloserine is considered to be the active form of the drug, having its action associated with the ability to inhibit two key enzymes, D-alanine racemase and D-alanine ligase. D-Alanine is an important component of the peptidoglycan portion of the mycobacterial cell wall. Mycobacterium are capable of utilizing natural occurring L-alanine and converting the L-alanine to D-alanine via the enzyme D-alanine racemase. The resulting D-alanine is coupled with itself to form a D-alanine–D-alanine complex under the influence of D-alanine ligase, and this complex is incorporated into the peptidoglycan of the mycobacterial cell wall . D-Cycloserine is a rigid analogue of D-alanine; therefore, it competitively inhibits the binding of D-alanine to both of these enzymes and its incorporation into the peptidoglycan. Resistance is associated with an over expression of D-alanine racemase.Фармаколо?гия
Cycloserine is readily absorbed orally and distributes throughout body fluids including the cerebrospinal fluid. The concentrations of cycloserine in tissues, body fluids, and the cerebrospinal fluid are approximately equal to the plasma level. Cycloserine is partially metabolized, and 60 to 80% is excreted unchanged by the kidney.Клиническое использование
D-(+)-4-Amino-3-isoxazolidinone (Seromycin) is an antibioticthat has been isolated from the fermentation beer of threedifferent Streptomyces species: S. orchidaceus, S. garyphalus,and S. lavendulus. It occurs as a white to pale yellow crystallinematerial that is very soluble in water. It is stable in alkaline,but unstable in acidic, solutions. The compoundslowly dimerizes to 2,5-bis(aminoxymethyl)-3,6-diketopiperazinein solution or standing.The structure of cycloserine was reported simultaneouslyby Kuehl et al. and Hidy et al.81 to be D-( +)-4-amino-3-isoxazolidinone. It has been synthesized byStammer et al. and by Smart et al.83 Cycloserine is stereochemicallyrelated to D-serine. However, the L-form hassimilar antibiotic activity.
Although cycloserine exhibits antibiotic activity invitro against a wide spectrum of both Gram-negative andGram-positive organisms, its relatively weak potency andfrequent toxic reactions limit its use to the treatment of tuberculosis.It is recommended for patients who fail to respondto other tuberculostatic drugs or who are known tobe infected with organisms resistant to other agents. It isusually administered orally in combination with otherdrugs, commonly isoniazid.
Побочные эффекты
Cycloserine is readily absorbed after oral administration and is widely distributed, including the CNS. Unfortunately, D-cycloserine binds to neuronal N-methylasparate receptors and, in addition, affects synthesis and metabolism of γ-aminobutyric acid, leading to complex series of CNS effects. As a second-line agent, cycloserine should only be used when retreatment is necessary or when the organism is resistant to other drugs. Cycloserine should not be used as a single drug; it must be used in combination.Методы очистки
Purify cycloserine by recrystallisation from aqueous EtOH or MeOH or aqueous NH3/EtOH or isoPrOH. Also recrystallise it from aqueous ammoniacal solution at pH 10.5 (100mg/mL) by diluting with 5 volumes of isopropanol and then adjusting to pH 6 with acetic acid. An aqueous solution, buffered to pH 10 with Na2CO3, can be stored in a refrigerator for 1week without decomposition. UV: max at 226nm (A1cm 1% 4.02). The tartrate salt has m 165-166o (dec), 166-168o (dec), and [] D 24 -41o (c 0.7, H2O). [Stammer et al. J Am Chem Soc 79 3236 1959, UV: Kuehl J Am Chem Soc 77 2344 1955, Beilstein 27 III/IV 5549.]Д-циклосерин запасные части и сырье
сырьё
1of3
запасной предмет
Д-циклосерин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 0755-36694831 +8613717124449 |
China | 300 | 58 | ||
| +8613586099526 | China | 217 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| 0523-86132544 | CHINA | 305 | 60 | ||
| +86-86-02137122233 +8613795318958 |
China | 299 | 55 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7738 | 55 |