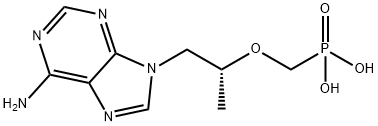
Тенофовир
- английское имяTenofovir
- CAS №147127-20-6
- CBNumberCB8218508
- ФормулаC9H14N5O4P
- мольный вес287.21
- EINECS604-571-2
- номер MDLMFCD07357269
- файл Mol147127-20-6.mol
| Температура плавления | 276-280°C |
| альфа | D +21° (c = 1 in 0.1M HCl) |
| Температура кипения | 616.1±65.0 °C(Predicted) |
| плотность | 1.79±0.1 g/cm3(Predicted) |
| температура хранения | -20°C |
| растворимость | Aqueous Acid (Sparingly), DMSO (Slightly, Heated), Water (Slightly, Heated) |
| пка | 2.36±0.10(Predicted) |
| форма | powder |
| цвет | white to beige |
| оптическая активность | [α]/D -20 to -26°, c = 0.5 in 1 M HCl |
| Растворимость в воде | 13.4 mg/mL (25 ºC) |
| Мерк | 14,9146 |
| InChI | InChI=1S/C9H14N5O4P/c1-6(18-5-19(15,16)17)2-14-4-13-7-8(10)11-3-12-9(7)14/h3-4,6H,2,5H2,1H3,(H2,10,11,12)(H2,15,16,17)/t6-/m1/s1 |
| ИнЧИКей | SGOIRFVFHAKUTI-ZCFIWIBFSA-N |
| SMILES | P(CO[C@H](C)CN1C2C(N=C1)=C(N)N=CN=2)(=O)(O)O |
| Справочник по базе данных CAS | 147127-20-6(CAS DataBase Reference) |
| FDA UNII | W4HFE001U5 |
| Словарь наркотиков NCI | tenofovir |
| UNSPSC Code | 51111800 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
H332:Вредно при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Тенофовир химические свойства, назначение, производство
Описание
Tenofovir is an analog of adenosine monophosphate that has antiviral activity. It is converted by cellular enzymes to tenofovir diphosphate, an obligate chain terminator that inhibits the activity of HIV reverse transcriptase and hepatitis B virus polymerase. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases α and β and mitochondrial DNA polymerase γ. For in vivo and cell culture use, tenofovir is supplied as a water soluble prodrug in the form of tenofovir disoproxil (fumarate) , which increases the intracellular diphosphorylated compound >1,000-Химические свойства
White Crystalline SolidИспользование
Tenofovir is a drug used for the treatment of chronic heptatitis B as well as prevention and treatment of HIV/AIDS. It is a kind of nucleotide analog, acting as the reverse-transcriptase inhibitor (NtRTI). It inhibits the activity of HIV reverse transcriptase through competing with the natural substrate deoxyadenosine 5’-triphosphate, causing the termination of DNA chain.Определение
ChEBI: A member of the class of phosphonic acids that is methylphosphonic acid in which one of the methyl hydrogens is replaced by a [(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy group. An inhibitor of HIV-1 reverse transcriptase, the bis(isopropyloxycarbonyloxy ethyl) ester (disoproxil ester) prodrug is used as the fumaric acid salt in combination therapy for the treatment of HIV infection.Показания
Tenofovir disoproxil fumarate (Viread) is a prodrug of tenofovir, a phosphorylated adenosine nucleoside analogue, and is the only available agent of its class. It is converted by cellular enzymes to tenofovir diphosphate, which competes with deoxyadenosine triphosphate (dATP) for access to reverse transcriptase and causes chain termination following its incorporation. Tenofovir was approved as part of a combination therapy for HIV in adults who failed treatment with other regimens; it appears to be effective against HIV strains that are resistant to NRTIs.Приобретенная устойчивость
HIV variants with the K65R mutation and the K70E mutation in the reverse transcriptase demonstrate reduced susceptibility to tenofovir.Фармацевтические приложения
A nucleotide analog structurally similar to adefovir.EC50 values for HBV, assessed in the HepG2 2.2.15 cell line, ranged from 0.14 to 1.5 μm; the cytotoxic concentration exceeded 100 μm. A decline in HBV DNA levels below 105 copies/mL at 48 weeks of therapy in 100% of patients receiving tenofovir compared with 44% on adefovir therapy has been reported. There are also case reports of patients with primary resistance to adefovir responding to tenofovir.
It is generally well tolerated in patients with chronic HBV; the most common side effects include nausea and gastrointestinal upset, headache, dizziness, fatigue and rash.
Биологическая активность
Selectively inhibits HIV reverse transcriptase (RNA-dependent DNA polymerase). Prevents cytotoxicity in SIV-infected C-8166 cells in vitro (IC 50 = 1.5 μ M). Antiviral agent.Фармакокине?тика
Oral absorption: c. 25%Cmax 300 mg once daily: 0.3 mg/L
Plasma half-life: 17 h
Volume of distribution: 1.3 ± 0.6 L/kg at 3.0 mg/kg intravenous dose
Plasma protein binding: <0.7% (in vitro)
Absorption and distribution
Oral bioavailability is poor, but is enhanced by administration as the disoproxil prodrug. It may be taken with or without food. CSF penetration is likely to be minimal due to the anionic charge of the molecule at physiological pH. It accumulates in semen at higher concentrations than in plasma. It is not known if it is distributed into breast milk.
Metabolism and excretion
Tenofovir is not metabolized and is principally eliminated by the kidneys by a combination of glomerular filtration and active tubular secretion. In patients with renal dysfunction the dose should be adjusted accordingly.
Compounds such as cidofovir, aciclovir (acyclovir), valaciclovir, ganciclovir, valganciclovir and probenecid may compete for renal excretion. Tenofovir levels are increased when prescribed with some HIV protease inhibitors. The co-administration of tenofovir with didanosine leads to didanosine accumulation which is thought to occur through inhibition of purine nucleoside phosphorylase. This has been associated with impaired immune recovery and several cases of lactic acidosis and pancreatitis. If tenofovir is combined with didanosine the dose of didanosine should be reduced to 200 mg (<60 kg) or 250 mg (≥60 kg) per day and the patient monitored for symptoms of didanosine toxicity.
Клиническое использование
Chronic hepatitis B infectionПобочные эффекты
In clinical trials of antiretroviral treatment-naive participants, the most commonly reported adverse events were mild to moderate gastrointestinal upset (nausea 8%, diarrhea 11%), headache (14%) and depression (11%). Tenofovir has the potential to result in nephrotoxicity, particularly through proximal tubular damage, but the risk of clinically significant renal dysfunction appears relatively low and seems to occur mainly in subjects with other identifiable risks for renal impairment. Minor elevations in serum creatinine and reductions in creatinine clearance occur, but rarely require drug discontinuation.A few (<0.1%) cases of osteomalacia and decreased bone density have been reported.
Тенофовир запасные части и сырье
Тенофовир поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 21-33585366 | CHINA | 1319 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| 025-84209270 15906146951 | CHINA | 115 | 55 | ||
| 010-60279497 | CHINA | 1803 | 55 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7738 | 55 | ||
| +86-21-33585366 - 03@ | CHINA | 738 | 60 |