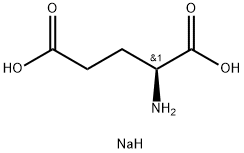
L-Glutamic acid monosodium salt monohydrate
- английское имяL-(+)Sodium glutamate
- CAS №142-47-2
- CBNumberCB8136677
- ФормулаC5H10NNaO4
- мольный вес171.13
- EINECS205-538-1
- номер MDLMFCD00013074
- файл Mol142-47-2.mol
химическое свойство
| Температура плавления | 232°C |
| альфа | 20 -3.5° (10% soln in 5° Bé HCl); D20 +25.16° (10g MSG/100ml 2N HCl) |
| плотность | d20 (saturated water soln): 1.620 |
| показатель преломления | 25 ° (C=10, 2mol/L HCl) |
| FEMA | 2756 | MONOSODIUM GLUTAMATE |
| температура хранения | Keep in dark place,Inert atmosphere,Room temperature |
| растворимость | Soluble in water; sparingly soluble in ethanol (95%). |
| форма | powder |
| цвет | white to off-white |
| Запах | Odorless |
| Биологические источники | synthetic |
| Растворимость в воде | >=10 g/100 mL at 20 ºC |
| Мерк | 6254 |
| Стабильность | Hygroscopic |
| LogP | -1.44 |
| FDA 21 CFR | 184.1321 |
| Вещества, добавляемые в пищу (ранее EAFUS) | MONOSODIUM GLUTAMATE |
| Специальный комитет по веществам GRAS | Monosodium L-glutamate |
| Справочник по базе данных CAS | 142-47-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | C3C196L9FG |
| Система регистрации веществ EPA | Monosodium glutamate (142-47-2) |
| UNSPSC Code | 85151701 |
| NACRES | NA.75 |
больше
| Заявления о рисках | 20/21/22 |
| WGK Германия | 2 |
| RTECS | MA1578000 |
| TSCA | Yes |
| кода HS | 29224999 |
| Банк данных об опасных веществах | 142-47-2(Hazardous Substances Data) |
| Токсичность | LD50 i.g. in mice: 19.9 g/kg (Eka) |
L-Glutamic acid monosodium salt monohydrate химические свойства, назначение, производство
Химические свойства
Colorless powderВхождение
Reported found in certain seaweeds including Laminaria japonicaИспользование
Flavor enhancer for foods in concentration of about 0.3%.Подготовка
Monosodium glutamate is commonly produced by a fermentation process using glucose (often sugar molasses) as a starting substance. Once the glucose is converted to glutamic acid, it is filtered, dissolved and converted to monosodium glutamate by neutralization with sodium hydroxide. The monosodium glutamate solution is decolorized and then crystallized, dried, sieved, packed and shippedМетоды производства
Monosodium glutamate is the monosodium salt of the naturally occurring L-form of glutamic acid. It is commonly manufactured by fermentation of carbohydrate sources such as sugar beet molasses. In general, sugar beet products are used in Europe and the USA. M 452 Monosodium Glutamate Other carbohydrate sources such as sugar cane and tapioca are used in Asia.Определение
ChEBI: An optically active form of monosodium glutamate having L-configuration.Общее описание
White or off-white crystalline powder with a slight peptone-like odor. pH (0.2% solution)7.0.Реакции воздуха и воды
Water soluble.Профиль реактивности
L-(+)Sodium glutamate is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).Пожароопасность
Flash point data are not available for L-(+)Sodium glutamate, but L-(+)Sodium glutamate is probably combustible.Фармацевтические приложения
Monosodium glutamate is used in oral pharmaceutical formulations as a buffer and a flavor enhancer. For example, it is used with sugar to improve the palatability of bitter-tasting drugs and can reduce the metallic taste of iron-containing liquids. It has also been used in subcutaneous live vaccine injections such as measles, mumps, rubella and varicella-zoster live vaccine (ProQuad). However, the most widespread use of monosodium glutamate is as a flavor enhancer in food products. Typically, 0.2–0.9% is used in normally salted foods, although products such as soy protein can contain 10–30%. The use of monosodium glutamate in food products has been controversial owing to the apparently high number of adverse reactions attributed to the substance, which gives rise to the so-called ‘Chinese Restaurant Syndrome’.The current consensus is that there is no clinically compelling evidence to suggest that monosodium glutamate may be harmful at the current levels used in foods.
Профиль безопасности
Moderately toxic by intravenous route. Mildly toxic by ingestion and other routes. An experimental teratogen. Other experimental reproductive effects. Human systemic effects by ingestion and intravenous routes: somnolence, hallucinations and distorted perceptions, headache, dyspnea, nausea or vomiting, dermatitis. The cause of "Chnese restaurant syndrome." When heated to decomposition it emits toxic fumes of NOx and Na2O.Безопасность
Monosodium glutamate is widely used in foods and oral pharmaceutical formulations. It is generally regarded as moderately toxic on ingestion or intravenous administration. Adverse effects include somnolence, hallucinations and distorted perceptions, headache, dyspnea, nausea or vomiting, and dermatitis. The lowest lethal oral dose in humans is reported to be 43 mg/kg.The use of monosodium glutamate in foods has been controversial due to the so-called ‘Chinese Restaurant Syndrome’, although it is generally regarded as safe at intake levels of up to 6 mg/kg bodyweight.In Europe, total glutamate intake from food ranges from 5–12 g/day.There has been a report of a foreign body granuloma caused by monosodium glutamate after a BCG vaccination.
Экологическая судьба
MSG is a white, odorless powder with high water solubility. If released into the air, it remains in the particulate phase until removed by deposition.хранилище
Aqueous solutions of monosodium glutamate may be sterilized by autoclaving. Monosodium glutamate should be stored in a tight container in a cool, dry place.Регуляторный статус
GRAS listed. Accepted in Europe for use as a food additive in certain applications. Included in the FDA Inactive Ingredients Database (oral syrup). Included in nonparenteral medicines licensed in the UK. Included in subcutaneous vaccine injections.L-Glutamic acid monosodium salt monohydrate запасные части и сырье
сырьё
1of3
запасной предмет
L-Glutamic acid monosodium salt monohydrate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +8617756083858 | China | 973 | 58 | |
| +8613031735486 | China | 105 | 58 | |
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | |
| +8615350851019 | China | 1001 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 |