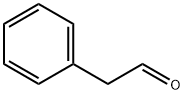
Фенилацетальдегид
- английское имяPhenylacetaldehyde
- CAS №122-78-1
- CBNumberCB7852954
- ФормулаC8H8O
- мольный вес120.15
- EINECS204-574-5
- номер MDLMFCD00006993
- файл Mol122-78-1.mol
| Температура плавления | −10 °C(lit.) |
| Температура кипения | 195 °C |
| плотность | 1.079 g/mL at 20 °C |
| давление пара | 2.09hPa at 20℃ |
| показатель преломления | n |
| FEMA | 2874 | PHENYLACETALDEHYDE |
| Fp | 188 °F |
| температура хранения | 2-8°C |
| растворимость | 2.21g/l slightly soluble |
| форма | Liquid |
| цвет | Clear colorless to pale yellow |
| Удельный вес | 1.075 (20/4℃) |
| Запах | at 10.00 % in dipropylene glycol. green sweet floral hyacinth clover honey cocoa |
| Odor Type | green |
| Биологические источники | synthetic |
| Растворимость в воде | 2.210 g/L (25 ºC) |
| Чувствительный | Air Sensitive |
| Мерк | 14,7265 |
| Номер JECFA | 1002 |
| БРН | 385791 |
| Диэлектрическая постоянная | 4.8(20℃) |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| LogP | 1.44 at 25℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | PHENYLACETALDEHYDE |
| Справочник по базе данных CAS | 122-78-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1-2 |
| FDA UNII | U8J5PLW9MR |
| Справочник по химии NIST | Benzeneacetaldehyde(122-78-1) |
| Система регистрации веществ EPA | Phenylacetaldehyde (122-78-1) |
| UNSPSC Code | 12352114 |
| NACRES | NA.22 |
| Коды опасности | Xn,F | |||||||||
| Заявления о рисках | 22-36/37/38-43-11 | |||||||||
| Заявления о безопасности | 26-36-37-24-16-7 | |||||||||
| РИДАДР | UN 1170 3/PG 2 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | CY1420000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29122990 | |||||||||
| Токсичность | LD50 orl-rat: 1550 mg/kg FCTXAV 17,377,79 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Фенилацетальдегид химические свойства, назначение, производство
Описание
Phenylacetaldehyde is an organic compound which can be naturally found in buckwheat, flowers, and communication pheromones from various insect orders. It is commonly used for the preparation of fragrance as well as flavors, and applied in flavored cigarettes and beverages because of its honey-like sweet character. It is also applied in the synthesis of polymers, such as polyesters, as a rate controlling additive in polymerization reactions and used in the preparation of more complex chemicals like resmethrin, where it acts as a building block.Химические свойства
Phenylacetaldehyde has a harsh, green odor reminiscent of hyacinth on dilution. It has an unpleasant, pungent, bitter flavor, turning sweet and fruit-like at low levels. Since it readily undergoes oxidation and polymerizes, it must be stabilized by addition of antioxidants and by dilution with, for example, diethyl phthalate before use in compositions.Вхождение
Identified among the constituents of several essential oils: neroli, Citrus sinensis leaves, other citrus species (flowers and leaves), narcissus, magnolia, lily, rose and tea. It is reported found in over 170 natural products including apricot, sour cherry, cooked apple, peach, fresh blackberry, crispbread, other types of bread, green tea, unprocessed rice, lemon balm, red sage, black currant, bilberry, cranberry, other berries, grapes, raisins, melon, papaya, guava fruit, pineapple, asparagus, celery leaves, carrot, parsley, peas, bell pepper, sweet pepper, peach, cabbage, peppermint oil, Scotch spearmint oil, mustard, vinegar, onion, cooked potato, tomato, cinnamon bark, cassia leaf, ginger, many cheeses, milk, yogurt, boiled egg, cooked and cured meats, beer, cognac, grape wines, cocoa, coffee, tea, roasted filbert, roasted peanut, soybean, pecans, cauliflower, broccoli, honey, avocado, passion fruit, beans, mushrooms, trassi, mango, tamarind, rice, licorice, buckwheat, lovage root, pumpkin, sweet potato, cassava, corn oil, malt, wort, dried bonito, loquat, pawpaw, maté, sweet grass oil, orange peel oil, grapefruit juice, endive, clam and Chinese quince.Использование
Phenylacetaldehyde is used for the preparation of fragrances and polymers like polyesters, which find application as a rate controlling additive in polymerization reactions. It is an active component of fragrances and floral scent due to its honey-like sweet character and finds application in flavored cigarettes and beverages. It is an insect attractant and utilized in blacklight trap for pests. It is also used as a building block in the synthesis of more complex chemicals, such as resmethrin. Furthermore, it is used in association with acetic anhydride and allyltrimethylsilane in three-component coupling process catalyzed by LiBF4 providing homoallylic acetates.Определение
ChEBI: Phenylacetaldehyde is an aldehyde that consists of acetaldehyde bearing a methyl substituent; the parent member of the phenylacetaldehyde class of compounds. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is an alpha-CH2-containing aldehyde and a member of phenylacetaldehydes.Общее описание
Phenylacetaldehyde is an important aroma volatile found in tomato and roses. It has also been identified in potato, roasted cocoa beans and honey. Phenylacetaldehyde is also a potent moth attractant.Профиль безопасности
Moderately toxic by ingestion. Human skin irritant. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES.Методы производства
The most important method industrially is the isomerization of styrene oxide. Ion-exchange resins, catalysis by chromium trioxide–tungsten trioxide on graphite or silicon dioxide–aluminum trioxide, and thermolysis are recommended for the rearrangement. Phenylacetaldehyde can also be synthesized by the direct oxidation of styrene in the presence of palladium salts and copper(II) chloride in aqueous solutions of glycol ethers. Other possibilities include the catalytic dehydrogenation of 2-phenylethanol on silver or gold catalysts, the hydroformylation of benzyl halides in the presence of dicobalt octacarbonyl and sodium carbonate in acetonitrile, and the Darzens glycide ester synthesis from benzaldehyde and alkyl chloroacetates.Синтез
Phenylacetaldehyde can be synthesized by Darzen glycidic ester synthesis from benzaldehyde; readily oxidizable to phenyl acetic acid.Фенилацетальдегид запасные части и сырье
сырьё
1of2
запасной предмет
- 2-фенил-2-бутенал
- 2-AMINO-5-PHENYL-THIOPHENE-3-CARBOXYLIC ACID METHYL ESTER
- Фенилацетальдегид диметил ацетал
- 2-Methyl-6-phenyl-4H-thieno[2,3-d][1,3]oxazin-4-one ,97%
- L-фенилаланин
- Cocal
- Бендрофлуметиазид
- 2-AMINO-5-PHENYL-3-THIOPHENECARBOXAMIDE
- 6-ФЕНИЛ-3H-ТИЕНО[2,3-D]ПИРИМИДИН-4-ОН
- Атропальдегид
1of4
Фенилацетальдегид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +8615318402391 | China | 809 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 025-83697070 | CHINA | 3009 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 |
Фенилацетальдегид Обзор)
- Фенилацетилен
- Фенилацетальдегид диметил ацетал
- Фенилацетил хлорид
- 1-(4-хлорфенил)-1-циклопропанкарбоновая кислота
- 2-бром-2-фенилацетофенон
- 1-Phenylcyclopentanecarboxylic кислота
- Хлорид Desyl
- Фенатренхинон
- 1-(4-хлорфенил)-1-циклопентанкарбоновая кислота
- 4-Fluorophenylacetone
1of4