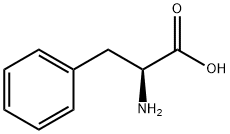
L-фенилаланин
- английское имяL-Phenylalanine
- CAS №63-91-2
- CBNumberCB2488691
- ФормулаC9H11NO2
- мольный вес165.19
- EINECS200-568-1
- номер MDLMFCD00064227
- файл Mol63-91-2.mol
| Температура плавления | 270-275 °C (dec.)(lit.) |
| альфа | -34.1 º (c=2, water, dry basis) |
| Температура кипения | 293.03°C (rough estimate) |
| плотность | 1.29 |
| Плотность накопления | 580kg/m3 |
| давление пара | <1 Pa (25 °C) |
| FEMA | 3585 | L-PHENYLALANINE |
| показатель преломления | -34 ° (C=2, H2O) |
| температура хранения | Store below +30°C. |
| растворимость | H2O: 0.1 M at 20 °C, clear, colorless |
| форма | powder |
| пка | 2.2(at 25℃) |
| цвет | White to off-white |
| РН | 5.0-7.0 (25℃, 0.1M in H2O) |
| Запах | at 100.00 %. odorless |
| Odor Type | odorless |
| Биологические источники | synthetic |
| оптическая активность | [α]25/D -34.2°, c = 2 in H2O (dried basis) |
| Растворимость в воде | 1-5 g/100 mL at 25 ºC |
| Номер JECFA | 1428 |
| Мерк | 14,7271 |
| БРН | 1910408 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | COLNVLDHVKWLRT-QMMMGPOBSA-N |
| LogP | 0.24 |
| Вещества, добавляемые в пищу (ранее EAFUS) | L-PHENYLALANINE |
| FDA 21 CFR | 172.320; 310.545 |
| Справочник по базе данных CAS | 63-91-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 47E5O17Y3R |
| Справочник по химии NIST | L-Phenylalanine(63-91-2) |
| Система регистрации веществ EPA | L-Phenylalanine (63-91-2) |
| Поглощение | cut-off at 274nm in H2O at 0.1M |
| UNSPSC Code | 41116107 |
| NACRES | NA.26 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 36/37/38-34 | |||||||||
| Заявления о безопасности | 22-24/25-37/39-45-36/37/39-27-26 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | AY7535000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29224995 | |||||||||
| Банк данных об опасных веществах | 63-91-2(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
L-фенилаланин химические свойства, назначение, производство
Химические свойства
L-Phenylalanine has no odor and a slight bitter taste. It melts with decomposition at about 283°C. The pH of a 1 in 100 solution is between 5.4 and 6.0. FEMA notes this chemical is used in cocoa substitute only.Вхождение
Reported found in white bread, macaroni, egg noodles, corn flakes, corn grits, oatmeal, wheat bran, wheat flakes, shredded wheat, barley, brown rice, rye flour, whole grain wheat flour, buttermilk, blue cheese, cheddar cheese, cottage cheese, cream cheese, parmesan cheese, bacon, cured ham, frankfurters, pork sausage, canned red kidney beans, canned sweet corn, canned peas, canned lima beans, canned potatoes, canned asparagus, canned snap beans, canned beets, beef, lamb, fresh ham, veal round, beef liver, chicken, chicken liver, turkey and other natural sources.Использование
L-Phenylalanine is an essential amino acid. L-Phenylalanine is biologically converted into L-tyrosine, another one of the DNA-encoded amino acids, which in turn is converted to L-DOPA and further conv erted into dopamine, norepinephrine, and epinephrine. L-Phenylalanine is produced for medical, feed, and nutritional applications such as in the preparation of Aspartame.Методы производства
In previous large-scale production processes for L-phenylalanine two enzymatic methods were applied: 1. Resolution of N-acetyl-D,L-phenylalanine by carrier-fixed microbial acylase: This process provided pharmaceutical-grade L-phenylalanine, but suffered from the disadvantage that the D-enantiomer had to be racemized and recycled. 2. Stereoselective and enantioselective addition of ammonia to trans-cinnamic acid, catalyzed by L-phenylalanine ammonia lyase (PAL, EC 4.3.1.5): PAL-containing Rhodotorula rubra was used in an industrial process to supply L-phenylalanine for the first production campaign of the sweetener aspartame. When continuously operated in an immobilized whole cell reactor, the bioconversion reached concentration up to 50 g/L Lphenylalanine at a conversion of about 83%. Other processes started from phenylpyruvate with L-aspartic acid as amine donor using immobilized cells of Escherichia coli or from a-acetamidocinnamic acid and immobilized cells of a Corynebacterium equi strain. In both cases L-phenylalanine concentrations up to 30 g/L and more (molar yields as high as at least 98 %) were reached.However, fermentation processes based on glucose-consuming L-phenylalanine overproducing ;mutants of E. coli and coryneform strains turned out to be more economical. L-Phenylalanine is formed in ten enzymatic steps starting from erythrose-4-phosphate and phosphoenolpyruvate. A suitable profile of the specific glucose feed rate prevents acetate formation and leads to improved L-phenylalanine production with a final concentration up to 46 g/L and a corresponding yield of 18 %.
L-Phenylalanine is recovered from the fermentation broth either by two-step crystallization or by an ionexchange resin process. The preferred cell separation technique is ultrafiltration; and the filtrates may be treated with activated carbon for further purification. Instead of ion-exchange resins nonpolar, highly porous synthetic adsorbents are recommended to remove impurities. An alternative process in which a cell separator is integrated in the fermentation part, thus allowing cell recycling, was suggested for L-phenylalanine production and may lead to prospective developments.
Подготовка
From PTS-negative Escherichia coli bioengineered strains.Определение
ChEBI: The L-enantiomer of phenylalanine.Общее описание
Odorless white crystalline powder. Slightly bitter taste. pH (1% aqueous solution) 5.4 to 6.Реакции воздуха и воды
Water soluble. Aqueous solutions are weak acids.Профиль реактивности
L-Phenylalanine may be light sensitive. Act as weak acids in solution.Угроза здоровью
ACUTE/CHRONIC HAZARDS: When heated to decomposition L-Phenylalanine emits toxic fumes of nitrogen oxides.Пожароопасность
Flash point data for L-Phenylalanine are not available; however, L-Phenylalanine is probably combustible.Профиль безопасности
Mildly toxic by intraperitoneal route. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.Методы очистки
Likely impurities are leucine, valine, methionine and tyrosine. Crystallise L-phenylalanine from water by adding 4volumes of EtOH. Dry it in vacuo over P2O5. Also crystallise it from saturated refluxing aqueous solutions at neutral pH, or 1:1 (v/v) EtOH/water solution, or conc HCl. It sublimes at 176-184o/0.3mm with 98.7% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2156-2175 1961, Beilstein 14 IV 1552.]L-фенилаланин запасные части и сырье
сырьё
1of4
запасной предмет
1of2
L-фенилаланин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +86-86-18148706580 +8618826483838 |
China | 147 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 |