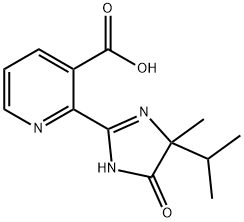
Imazapyr acid
- русский язык имя
- английское имяImazapyr acid
- CAS №81334-34-1
- CBNumberCB7121753
- ФормулаC13H15N3O3
- мольный вес261.28
- EINECS617-219-8
- номер MDLMFCD00144470
- файл Mol81334-34-1.mol
химическое свойство
| Температура плавления | 169-173°C |
| Температура кипения | 404.53°C (rough estimate) |
| плотность | 1.1923 (rough estimate) |
| давление пара | 0-0Pa at 20-25℃ |
| показатель преломления | 1.5600 (estimate) |
| температура хранения | 0-6°C |
| пка | 1.9, 3.6(at 25℃) |
| БРН | 5442754 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| LogP | -3.97-0.04 at 20℃ and pH3-9.9 |
| Dissociation constant | 1.7-11.1 at 20℃ |
| Справочник по базе данных CAS | 81334-34-1(CAS DataBase Reference) |
| FDA UNII | 787MX0M5A6 |
| Система регистрации веществ EPA | Imazapyr (81334-34-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xi |
| Заявления о рисках | 36-52/53 |
| Заявления о безопасности | 26-61 |
| WGK Германия | 2 |
| RTECS | US5682500 |
| кода HS | 29333990 |
| Банк данных об опасных веществах | 81334-34-1(Hazardous Substances Data) |
| Токсичность | LD50 orally in rats: >5000 mg/kg; dermally in rabbits: >2000 mg/kg (Paxman) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
Imazapyr acid химические свойства, назначение, производство
Химические свойства
solidИспользование
Imazapyr is an analytical standard used for proteomics research.Фармаколо?гия
Imazapyr kills plants by inhibiting acetolactate synthase (ALS) (I50 = 5 μM), which is the first common enzyme in the biosynthesis of the branched chain amino acids, valine, leucine, and isoleucine. Imazapyr is rapidly absorbed through the leaves of plants. Once it enters the plant, imazapyr rapidly translocates to the growing points and growth ceases within 1 day after herbicide application followed by chlorosis and then necrosis of the growing points. Total plant death will occur within 2 to 3 weeks after treatment.Экологическая судьба
Imazapyr is weakly to moderately adsorbed on sandy loam and silt loam soils. The Freundlich adsorption coefficient ranges from 0 to 7.8 (15). Because imazapyr is a weak acid and exists in different ionic states, soil pH has an effect on soil binding properties. The anionic form predominates at soil pH as low as 5.5, and this form bindsweakly to soil. The neutral or molecular form is important at soil pH from 4 to 6.5. This form binds to soil organic matter and clay. The cationic form is important at pH less than 4. Because the soil is a heterogeneous mixture of acid and base chemical groups, there may be sites within a particular soil that are 2 to 3 pH units higher or lower than the average pH. The cationic form will bind tightly to the lower pH components. Because of these interactions, small decreases in pH below 6 will result in large increases in binding. The half-life of imazapyr in the soil is 25–142 d (14). Imazapyr remains in the top 30 cm of the soil with low leaching potential. The degradation route of imazapyr in the soil has not been determined.Метаболизм
Plant Metabolism. The selectivity of imazapyr is due to differential rates and routes of metabolism in tolerant crops versus susceptible weeds (3). The half-life of imazapyr in tolerant crops has not been accurately determined. The metabolic route of imazapyr is not clear. The parent compound can be metabolized to a tricyclic compound (33, Fig. 15) in some species, but the primary metabolite is an imidazopyrrolo-pyridine derivative (34, Fig. 15). This compound does not inhibit acetolactate synthase, the target site for the imidazolinones, and it is immobile in the plant (4).Animal Metabolism. Metabolism studies in the rat showed that imazapyr is rapidly excreted in the urine (5). There was no accumulation of imazapyr or any of its derivatives in the liver, kidney, muscle, fat, or blood.
Imazapyr acid запасные части и сырье
Imazapyr acid поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | |
| 008657128800458; +8615858145714 |
China | 7782 | 55 | |
| +86-755-89396905 +86-15013857715 |
China | 10453 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 |