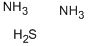
Аммоний сульфид
- английское имяAmmonium sulfide
- CAS №12135-76-1
- CBNumberCB6151876
- ФормулаH8N2S
- мольный вес68.14
- EINECS235-223-4
- номер MDLMFCD00010892
- файл Mol12135-76-1.mol
| Температура плавления | decomposes [HAW93] |
| Температура кипения | 40 °C |
| плотность | 1 g/mL at 25 °C |
| давление пара | 600 hPa at 20 °C |
| FEMA | 2053 | AMMONIUM SULFIDE |
| показатель преломления | n |
| Fp | 90 °F |
| температура хранения | room temp |
| растворимость | Very soluble in water, almost insoluble in alcohol and oils. |
| пка | 3.42±0.70(Predicted) |
| форма | Liquid |
| цвет | Yellow to orange |
| Удельный вес | 1.2 |
| Запах | Ammonia odour |
| Водородный показатель | 9.5 ( 45% aqueous solution) |
| РН | 9.5 (450g/L in H2O) |
| Биологические источники | synthetic |
| Odor Type | sulfurous |
| Пределы взрываемости | 4-46% |
| Растворимость в воде | Miscible with water, alcohol and liquid ammonia. |
| Мерк | 13,560 |
| Стабильность | Stable. Pure material is highly flammable - note wide explosion limits. Incompatible with strong oxidizing agents, strong acids, copper, brass, bronze, strong bases, zinc, aluminium. |
| ИнЧИКей | UXXGWUQNRMPNKH-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 12135-76-1(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | AMMONIUM SULFIDE |
| FDA 21 CFR | 172.515 |
| FDA UNII | 2H0Q32TDFZ |
| Система регистрации веществ EPA | Ammonium sulfide (12135-76-1) |
| UNSPSC Code | 12352301 |
| NACRES | SE.11 |
| Коды опасности | C,N,F | |||||||||
| Заявления о рисках | 11-31-34-50-10 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-61-16 | |||||||||
| РИДАДР | UN 2683 8/PG 2 | |||||||||
| WGK Германия | - | |||||||||
| F | 10-13-23 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28309090 | |||||||||
| Банк данных об опасных веществах | 12135-76-1(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Аммоний сульфид химические свойства, назначение, производство
Химические свойства
Ammonium sulfide is a yellow crystalline (sugar or sand-like) material, commonly found in liquid solution, which is flammable. Solution has an odor of rotten eggs.Физические свойства
Unstable, decomposes at ambient temperature; forms yellow crystals below -18°C; hygroscopic; soluble in water and alcohol, very soluble in liquid ammonia.Использование
Ammonium sulfide solution is used occasionally in photographic developing, to apply patina to bronze, and in textile manufacturing. Ammonium sulfide is a very useful reagent in mineral analysis. Used in continuous culture of saccharomyces cerevisiae, useful in a double staining technique for visualization of astrocytes and microglia from brain sections and astroglial cell cultures. It plays an important role to modify the surface of low κ dielectric thin films. It is essential for molecular beam epitaxy regrowth. It is an unstable salt and acts as a selective reducing agent.Общее описание
Ammonium sulfide aqueous solution is a colorless to yellow liquid, with an odor of rotten eggs or ammonia. Material in aqueous from the hydrosulfide which with acid forms H2S. Ammonium sulfide may be irritating to skin, eyes and mucous membranes and may cause illness from skin absorption. Ammonium sulfide may burn and/or emit toxic fumes if heated to high temperatures.Реакции воздуха и воды
Readily oxidized to be pyrophoric in air [Bretherick 1979 p. 120]. Ammonium sulfide is slowly decomposed by moisture giving off hydrogen sulfide, a flammable gas. Heat is generated when the pure compound is first dissolved in water.Профиль реактивности
AMMONIUM SULFIDE SOLUTION is a strongly alkaline aqueous solution. Reacts with acids to generate toxic gaseous hydrogen sulfide. Reacts with bases to release gaseous ammonia. May react vigorously with oxidizing agents, including inorganic oxoacids, organic peroxides and epoxides. Special Hazards of Combustion Products: Toxic hydrogen sulfide gas is released when solution is heated. If ignited, this will form irritating sulfur dioxide gas [USCG, 1999].Угроза здоровью
Inhalation of 500 ppm for 30 min. produces headaches, dizziness, bronchial pneumonia; 600 ppm for 30 min. can cause death. Ingestion causes severe irritation of mucous membranes and stomach. Contact with liquid causes severe burns of eyes and severe skin irritation. May be absorbed through skin and cause hydrogen sulfide poisoning.Пожароопасность
Special Hazards of Combustion Products: Toxic hydrogen sulfide gas is released when solution is heated. If ignited, this will form irritating sulfur dioxide gas.Промышленное использование
Ammonium sulfide (NH4)2S is a liquid with an obnoxious odor and because of this, it is not normally used in mineral processing. However, this is the most effective depressant for bornite and covellite. Essentially, (NH4)2S dissolves excess sulfur from the mineral surface, that allows other depressants to adsorb onto the mineral surface. In copper–lead separation, from a bulk concentrate containing covellite and bornite, (NH4)2S can effectively be used together with cyanide.Возможный контакт
It is used in photographic developers, synthetic flavors, coloring metals (i.e., to apply patina to bronze); and to make textiles.Перевозки
UN2923 Corrosive solids, toxic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous material, Technical Name Required. UN2683 Ammonium sulfide solution, Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous materials, 3-Flammable liquidНесовместимости
Vapor form explosive mixture with air Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Evolves poisonous ammonia on contact with strong bases. Contact with acid or acid fumes releases hydrogen sulfide. Keep away from moisture.Утилизация отходов
Add to a large volume of ferric chloride solution with stirring. Neutralize with soda ash. Flush to drain with water.Аммоний сульфид запасные части и сырье
Аммоний сульфид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 2039322489 | United States | 168 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 |