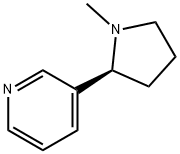
Никотин
- английское имяNicotine
- CAS №54-11-5
- CBNumberCB5293753
- ФормулаC10H14N2
- мольный вес162.23
- EINECS200-193-3
- номер MDLMFCD00006369
- файл Mol54-11-5.mol
| Температура плавления | -80 °C |
| альфа | -166 º (c=neat) |
| Температура кипения | 243-248 °C |
| плотность | 1.010 g/mL at 20 °C(lit.) |
| давление пара | 0.06 hPa (20 °C) |
| показатель преломления | n |
| Fp | 215 °F |
| температура хранения | 2-8°C |
| растворимость | ethanol: 50 mg/mL |
| пка | 8.02(at 25℃) |
| форма | liquid |
| цвет | yellow |
| РН | 10.2 (8.1g/l, H2O, 20°C) |
| Пределы взрываемости | 0.7-4%(V) |
| Порог?обнаружения?запаха? | 0.011ppm |
| оптическая активность | [α]20/D 169°(lit.) |
| Растворимость в воде | MISCIBLE |
| Чувствительный | Air Sensitive & Hygroscopic |
| Мерк | 14,6524 |
| БРН | 82109 |
| Пределы воздействия | TLV-TWA air 0.5 mg/m3 (ACGIH, MSHA, and OSHA). |
| LogP | 1.170 |
| Справочник по базе данных CAS | 54-11-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3-7 |
| Словарь онкологических терминов NCI | nicotine; nicotine gum; nicotine patch |
| FDA UNII | 6M3C89ZY6R |
| Словарь наркотиков NCI | nicotine |
| Код УВД | N07BA01 |
| Предложение 65 Список | Nicotine |
| Справочник по химии NIST | 3-(2-(N-methylpyrrolidinyl))pyridine(54-11-5) |
| Система регистрации веществ EPA | Nicotine (54-11-5) |
| UNSPSC Code | 12352210 |
| NACRES | NA.77 |
| Коды опасности | T+,N,Xn,F,Xi,T | |||||||||
| Заявления о рисках | 25-27-51/53-36-20/21/22-11-36/37/38-39/23/24/25-23/24/25-59-48/20-40-36/38-24-20/22-63 | |||||||||
| Заявления о безопасности | 7-16-36/37-45-61-36-26-37/39-59 | |||||||||
| РИДАДР | UN 1654 6.1/PG 2 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.5 mg/m3 [skin] | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | QS5250000 | |||||||||
| Температура самовоспламенения | 240 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29399910 | |||||||||
| Банк данных об опасных веществах | 54-11-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in mice (mg/kg): 0.3 i.v.; 9.5 i.p.; 230 orally (Barlow, McLeod) | |||||||||
| ИДЛА | 5 mg/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H318:При попадании в глаза вызывает необратимые последствия.
H411:Токсично для водных организмов с долгосрочными последствиями.
H300+H310+H330:Смертельно при проглатывании, при контакте с кожей или при вдыхании.
-
оператор предупредительных мер
P262:Избегать попадания в глаза, на кожу или одежду.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352+P310:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Немедленно обратиться за медицинской помощью.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Никотин химические свойства, назначение, производство
Описание
Nicotine is an alkaloid obtained from the dried leaves of Nicotiana tabacum and Nicotiana rustica. Nicotine stimulates acetylcholine receptors of the postsynaptic membrane at nerve synapses resulting in depolarization of the membrane. Toxic doses cause stimulation that is rapidly followed by blockade of nerve transmission.Химические свойства
Nicotine is a pale yellow to dark brown, oily liquid. Slight, fishy or pyridine-like odor when warm. It is also available as a powder.Использование
(S)-(-)-Nicotine is a prototype nicotinic acetylcholine receptor agonist. (S)-(-)-Nicotine is the naturally occurring isomer. Nicotine can be absorbed through the alimentary canal, respiratory tract and intact skin. Nicotine is used in the treatment of smoking withdrawal syndrome. Nicotine has been used as an anthelmintic.Определение
ChEBI: An optically active form of nicotine having S-configuration.Методы производства
The nicotine molecule consists of a pyrrolidine ring attached to a pyridine ring by a bondbetween carbon atoms in the two-ring systems. Nicotine was isolated in impure form fromtobacco in 1809 by Louis Nicholas-Vauquelin (1763–1829). Vauquelin called the substancenicotianine. In 1826, Wilhelm Posselt (1806–1877) and Karl Ludwig Reimann (1804–1872),medical students at Heidelberg University, isolated pure nicotine and published dissertationson its pharmacology in 1828. Louis Henri Melsens (1814–1886) determined nicotine’sempirical formula. Amé Pictet (1857–1937) and P. Crépieux reported the synthesis of nicotine in 1903.Общее описание
Liquids. Toxic by inhalation, ingestion, and skin absorption. More dense than water. Flash points usually below 140°F. Contact may irritate skin, eyes, and mucous membranes. If available, obtain the technical name of the material from the shipping papers and contact CHEMTREC, (800-424-9300) for specific response information.Реакции воздуха и воды
Flammable. Slightly soluble in water.Профиль реактивности
An alkaloid produced from tobacco. Colorless, oily liquid, combustible, highly toxic. When heated to decomposition L-Nicotine emits very toxic fumes of carbon monoxide and oxides of nitrogen [Lewis, 3rd ed., 1993, p. 919].Опасность
Toxic by ingestion, inhalation, and skin absorption. Gastrointestinal damage, central nervous system impairment, and cardiac impairmentУгроза здоровью
L-Nicotine is classified as super toxic. Probable oral lethal dose in humans is less than 5 mg/kg or a taste (less than 7 drops) for a 70 kg (150 lbs.) person. It may be assumed that ingestion of 40-60 mg of nicotine is lethal to humans. There is fundamental difference between acute toxicity from use of nicotine as insecticide or from ingestion, and chronic toxicity that may be caused by prolonged exposure to small doses as occurs in smoking. Maternal smoking during pregnancy is associated with increased risk of spontaneous abortion, low birth weight and still-birth. Nicotine was found as a co-carcinogen in animals.Пожароопасность
There is a moderate explosion hazard when exposed to heat or flame. When heated to decomposition, L-Nicotine emits nitrogen oxides, carbon monoxide and other highly toxic fumes. Avoid oxidizing materials. Stable under normal conditions. Avoid heat or flames.Сельскохозяйственное использование
Insecticide: Nicotine is used in some drugs and insecticides. Classified for restricted use as an insecticide, limited to use by or under the direct supervision of a certified applicator. Not listed for use in EU countries. Registered for use in the U.S. and Canada. A U.S. EPA restricted Use Pesticide (RUP).Торговое название
BLACK LEAF®; CAMPBELL'S NICOSOAP ®; DESTRUXOL ORCHARD SPRAY®; EMONIB ®; FLUX MAAG®; FUMETO-TENDUST®; BAC®; MACH-NIC®; NIAGARA P. A. DUST®; NICODUST®; NICOFUME®; NICOCIDE®; ORTHO N-4 DUST®; XL ALL INSECTICIDE®Возможный контакт
An alkaloid produced from tobacco. Nicotine is used in some drugs; and in tanning. At one time, nicotine was used in the United States as an insecticide and fumigant; however, it is no longer produced or used in the United States for this purpose.Канцерогенность
Nicotine has low carcinogenic potential. One study found that diets containing 60 ppm nicotine and administered to rats for 300 days reduced the growth rate. The authors concluded that reduced body weight gains were only partially attributable to reduced food intake. No pathology on the rats and no evidence of carcinogenicity were reported. Rats were injected subcutaneously (5 days/week) for 26 weeks followed by an approximate 2-month observation period. Similarly, dogs were injected subcutaneously (5 days/week) for the same period. No tumors were observed in the test animals, although hyaline thickening and fibrosis of the vasculature of the kidney, lung, brain, and heart were evident.Метаболический путь
Nicotine has been used as an insecticide for at least 200 years but this naturally occurring compound lacks persistence and can be hazardous in use (Corbett et al., 1984). It has been replaced with more effective synthetic insecticides such as those in the neonicotinoid class. Most of the mformation on metabolism derives from research into the fate of nicotine after tobacco smoking as well as from the use of nicotine in agriculture and horticulture or through the biosynthesis of the alkaloid by plants and vegetables used as normal foodstuffs. Up to eight metabolites have been isolated and identified in man with six primary pathways. The main pathway is N-carbon oxidation of the pyrrolidine ring to form cotinine, others being N-oxidation of the pyrrolidine ring to form nicotine N-oxide, N-methylation of the pyridine ring to form an N-methylnicotinium ion and N-demethylation of the pyrrolidine ring to form nornicotine. Two other pathways are formation of a nicotine enamhe and of a nicotine glucuronide (Gabrielsson and Gumbleton, 1993). There is little information on the fate of nicotine in soil.Метаболизм
Nicotine is well absorbed from the mucous membranes in the oral cavity, gastrointestinal tract, and respiratory system. If tobacco smoke is held in the mouth for 2 seconds, 66 to 77% of the nicotine in the smoke will be absorbed across the oral mucosa. If tobacco smoke is inhaled, approximately 90 to 98% of the nicotine will be absorbed. Nicotine is distributed throughout the body, readily crossing the blood-brain and placental barriers. The liver, kidney, and lung metabolize approximately 80 to 90% of the alkaloid. The kidney rapidly eliminates nicotine and its metabolites.Перевозки
UN1654 Nicotine, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Методы очистки
(-)-Nicotine is a very pale yellow hygroscopic oil with a characteristic odour (tobacco extract) which turns brown in air on exposure to light. It is purifed by fractional distillation under reduced pressure in an inert atmosphere. A freshly distilled sample should be stored in dark sealed containers under N2. It is a strong base; a 0.05 M aqueous solution has a pH of 10.2. It is very soluble in organic solvents. It is soluble in H2O and readily forms salts. [UV: Parvis J Chem Soc 97 1035 1910, Dobbie & Fox J Chem Soc 103 1194 1913.] The hydrochlorides (mono-and di-) form deliquescent crystals soluble in H2O and EtOH but insoluble in Et2O. It has also been purified via the ZnCl2 double salt. [Ratz Monatsh Chem 26 1241 1905, Biosynthesis: Nakan & Hitchinson J Org Chem 43 3922 1978.] The picrate has m 218o (from EtOH). [Beilstein 23/6 V 64.] POISONOUS.Несовместимости
Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Incompatible with strong acids. Attacks some forms of plastics, rubber, and coatings.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.использованная литература
Pictet, Rotschy., Ber., 33, 2533 (1900)Tschitschibabin, Buchholz.,J. Russ. Phys. Chern. Soc., 50, 548 (1920)
Spath, Biniecki., Ber., 72, 1809 (1939)
Shmuk, Borozdina., CampI. rend. Acad. Sci., USSR, 12,1582 (1939)
Shmuk, Borozdina., J. Appl. Chern. Russ., 14,864 (1941)
Smith, Smith., J. Agric. Res., 65, 347 (1942)
Pal, Narasinham.,!. Ind. Chern. Soc., 20, 181 (1943)
Marion., Can. J. Res., 23B, 165 (1945)
Bottomley, Nottle, White., Austral. J. Sci., 8, 18 (1945)
Biosynthesis: Leete.,!. Amer. Chern. Soc., 89,7081 (1967)
14C-NMR spectrum: Ganz, Kelsey, Geiling., Bot. Gaz., 113, 195 (1951)
13C-NMR spectrum: Crain, Wilderman, Roberts., J. Amer. Chern. Soc., 93,990 (1971)
Pharmacology : Rolleston., Lancet., 210,961 (1926)
Laessing., Med. Welt., 12, 1485 (1938)
Coon, Rothman., Proc. Soc. Exp. Bioi. Med., 42, 231 (1939)
Straub, Amann., Klin. Woch., 19,169 (1940)
Coon, Rothman., J. Pharm. Exp. Ther. Froc., 72, 9 (1941)
Perlman, Dannesborg, Sokoloff., J. Amer. Med. Assoc., 120, 1003 (1942)
Roth, McDonald, Sheard., ibid, 125,761 (1944)
Burn, Truelove, Burn., Brit. Med. J., i, 403 (1945)