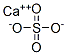
calcium sulfate
- русский язык имя
- английское имяcalcium sulfate
- CAS №99400-01-8
- CBNumberCB82130352
- ФормулаCaO4S
- мольный вес136.14
- файл Mol99400-01-8.mol
химическое свойство
| Температура плавления | >300°C |
| температура хранения | Hygroscopic, Room Temperature, under inert atmosphere |
| растворимость | Water (Slightly) |
| форма | Solid |
| цвет | Off-White to Pale Grey |
| Растворимость в воде | 2.4g/L |
| Константа произведения растворимости (Ksp) | pKsp: 4.31 |
| Диэлектрическая постоянная | 5.6(0.0℃) |
| FDA 21 CFR | 184.1230; 582.5230; 176.170; 178.3297 |
| Рейтинг продуктов питания EWG | 1 |
calcium sulfate химические свойства, назначение, производство
Использование
Calcium Sulfate is a general additive available as both calcium sul- fate anhydrous, made by the high-temperature calcining of gypsum which is then ground and separated, and calcium sulfate dihydrate, which is made by grinding and separating gypsum containing about 20% water of crystallization. calcium sulfate anhydrous contains approximately 29% calcium, and calcium sulfate dihydrate contains approximately 23% calcium. it is used, among other things, as a filler and baking powder for standardization purposes; a firming agent in canned potatoes, tomatoes, carrots, lima beans, and pep- pers; in dough as a source of calcium ions (because the absence of calcium ions causes bread dough to be soft and sticky and to pro- duce bread of poor quality); in soft-serve ice cream to produce dry- ness and stiffness; as a calcium ion source for reaction with alginates to form dessert gels; and as a calcium source for food enrichment.Подготовка
Calcium Sulfate may be prepared by the addition of a soluble calcium salt to a solution of sodium sulfate:CaCl2+ Na2SO4→CaSO4+ 2NaCl
The product is generally a dihydrate although other hydrates are also known. The CAS numbers of these materials are: 7778-18-9 (anhydrite); 10034-76-1 (hemihydrate); 10101-41-4 (dihydrate).
Промышленное использование
Calcium sulfate (CaSO4?2H2O) is the common blackboard chalk.calcium sulfate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | |
| +86-86-13583358881 +8618560316533 |
China | 3094 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| +86-27-87465837 +8618971612321 |
China | 9639 | 58 | |
| China | 12341 | 58 | ||
| +86-85511178; +86-85511178; |
China | 35425 | 58 | |
| +86-0512-83500002 +8615195660023 |
China | 23046 | 58 | |
| +8613063595538 | China | 9363 | 58 | |
| 027-87465837 19945049750 |
China | 9648 | 58 | |
| 010-50973186 4009686088 |
China | 18338 | 68 |