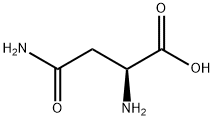
L-аспарагин
- английское имяL-Asparagine
- CAS №70-47-3
- CBNumberCB4375647
- ФормулаC4H8N2O3
- мольный вес132.12
- EINECS200-735-9
- номер MDLMFCD00064401
- файл Mol70-47-3.mol
химическое свойство
| Температура плавления | 235 °C (dec.) (lit.) |
| Температура кипения | 244.01°C (rough estimate) |
| альфа | 34.5 º (c=10, 2N HCl) |
| плотность | 1,543g/cm |
| показатель преломления | 1.4880 (estimate) |
| температура хранения | Keep in dark place,Inert atmosphere,Room temperature |
| растворимость | Practically insoluble in methanol, ethanol, ether, benzene. Soluble in acids and alkalies. |
| пка | 2.17(at 20℃) |
| форма | Powder |
| цвет | White |
| Запах | sltly sweet taste |
| РН | 5.41 |
| оптическая активность | -5.625 (H2O)+28.625 (1 mol dm-3 HCl) |
| Растворимость в воде | H2O: 20 g/L (20 oC) , clear, colorless |
| Чувствительный | Hygroscopic |
| Мерк | 14,837 |
| БРН | 1723527 |
| Стабильность | Stable, but may be moisture-sensitive. Incompatible with strong oxidizing agents. |
| LogP | -3.820 |
| Справочник по базе данных CAS | 70-47-3(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | L-ASPARAGINE |
| FDA 21 CFR | 172.320 |
| FDA UNII | 5Z33R5TKO7 |
| Справочник по химии NIST | L-Asparagine(70-47-3) |
| Система регистрации веществ EPA | L-Asparagine (70-47-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.75 |
больше
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 20/21/22-36/37/38 | |||||||||
| Заявления о безопасности | 24/25-36-26 | |||||||||
| РИДАДР | UN 2811 6.1 / PGIII | |||||||||
| WGK Германия | 3 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Y | |||||||||
| Класс опасности | IRRITANT | |||||||||
| кода HS | 29241900 | |||||||||
| Банк данных об опасных веществах | 70-47-3(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
L-аспарагин химические свойства, назначение, производство
Описание
Asparagine (abbreviated as Asn or N) is one of the 20 most common natural amino acids on Earth. It has carboxamide as the sidechain's functional group. It is not an essential amino acid. Its codons are AAU and AAC.The amino acid L-asparagine is a structural analog of L-aspartic acid, where the side chain of the carboxylic acid moiety is amidated, to give a terminal amine group. This renders L-asparagine neutral at physiological pH. The amide group of asparagine is derived from glutamate, in the reaction of aspartate and glutamine in the presence of ATP to yield asparagine and glutamate. In vivo, asparagine is hydrolyzed to aspartate and NH4+ by asparaginase. Asparagine is also an important amino acid in glycopeptide bonds, via N-glycosyl linkages to the sugar rings.
Химические свойства
White crystal or crystalline powder with a slightly sweet taste. Slightly soluble in water, insoluble in ethanol and ether, it often exists as a monohydrate, and it is a rhombic hemihedral crystals. The melting point is 234-235°C , and the aminocarbonyl reaction is carried out by co-heating with sugar, which can form special aroma substances. The best recrystallization method is water, followed by ethanol. In case of alkali hydrolysis into aspartic acid. Heating its aqueous solution also decomposes. Natural products exist in various legumes and the like.Физические свойства
Solubility 3.11 (28 ℃) g/100 g H2O, pI 5.41, dissociation constants: pK1 2.02, pK2 8.8.Вхождение
Dietary sourcesAsparagine is not essential for humans, which means that it can be synthesized from central metabolic pathway intermediates and is not required in the diet. Asparagine is found in :
Animal sources : dairy, whey, beef, poultry, eggs, fish, lactalbumin , sea food
Plant sources : asparagus, potatoes, legumes, nuts, seeds, soy, whole grains.
Biosynthesis
The precursor to asparagine is oxaloacetate. Oxaloacetate is converted to aspartate using a transaminase enzyme. The enzyme transfers the amino group from glutamate to oxaloacetate producing α- ketoglutarate and aspartate. The enzyme asparagine synthetase produces asparagine, AMP, glutamate, and pyrophosphate from aspartate, glutamine, and ATP. In the asparagine synthetase reaction, ATP is used to activate aspartate, forming β-aspartyl-AMP. Glutamine donates an ammonium group, which reacts with β-aspartyl-AMP to form asparagine and free AMP.
История
Asparagine was first isolated in 1806, under a crystalline form, by French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet (then a young assistant) from asparagus juice, in which it is abundant — hence, the name they chose for that new matter — becoming the first amino acid to be isolated.A few years later, in 1809, Pierre Jean Robiquet again identified, this time from liquorice root, a substance with properties he qualified as very similar to those of asparagine, that Plisson in 1828 identified as asparagine itself.
Использование
L-asparagine has been used:to identify and quantify free amino acids released upon oxidation of proteins and peptides by hydroxyl radicals.
to study the effects of amino acids in promoting food consumption in Drosophila melanogaster.
to study non-enzymatic gluconeogenesis.
L-Asparagine is used in cell culture media and is a component of MEM non-essential amino acids solution.
L-Asparagine has been shown to enhance ornithine decarboxylase activity in cultured human colon adenocarcinoma Caco-2 cells and in cultured IEC-6 intestinal epithelial cells. Spore germination in Bacillus subtilis has been increased in the presence of L-asparagine.
An isoxazoline RGD mimic platelet GPIIb/IIIa antagonist has been prepared by chiral synthesis with L-asparagine as a starting material. L-Asparagine has been utilized in the synthesis of 4-azalysine building blocks for application to combinatorial chemistry.
Определение
ChEBI: L-asparagine is an optically active form of asparagine having L-configuration. It has a role as a nutraceutical, a micronutrient, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and a plant metabolite. It is an aspartate family amino acid, a proteinogenic amino acid, an asparagine and a L-alpha-amino acid. It is a conjugate base of a L-asparaginium. It is a conjugate acid of a L-asparaginate. It is an enantiomer of a D-asparagine. It is a tautomer of a L-asparagine zwitterion.Методы производства
A simple synthesis of L -asparagine starts from L -aspartic acid which is esterified to the b-methyl ester followed by treatment with ammonia.Биологические функции
Asparagine is a dietarily dispensable amino acid synthesized from aspartate and glutamine. Asparagine has three major functions: 1) incorporation into amino acid sequences of proteins; 2) storage form for aspartate (is a required precursor for synthesis of DNA, RNA and ATP); and 3) source of amino groups for production of other dispensable amino acids via trasaminases.The nervous system requires asparagine. It also plays an important role in the synthesis of ammonia.
The addition of N-acetyl glucosamine to asparagine is performed by oligosaccharyltransferase enzymes in the endoplasmic reticulum. This glycosylation is important both for protein structure and protein function.
Профиль безопасности
When heated to decomposition emits toxic fumes of NoxМетоды очистки
Likely impurities are aspartic acid and tyrosine. Crystallise it from H2O or aqueous EtOH. It slowly effloresces in dry air. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 1856 1961, Beilstein 4 IV 3005.]L-аспарагин запасные части и сырье
L-аспарагин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8613063595538 | China | 9363 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | ||
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 |