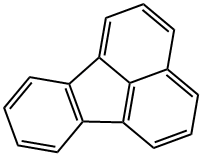
Фторантен
- английское имяFluoranthene
- CAS №206-44-0
- CBNumberCB3123401
- ФормулаC16H10
- мольный вес202.25
- EINECS205-912-4
- номер MDLMFCD00001184
- файл Mol206-44-0.mol
| Температура плавления | 105-110 °C (lit.) |
| Температура кипения | 384 °C (lit.) |
| плотность | 1.252 |
| показатель преломления | 1.0996 |
| Fp | -18 °C |
| температура хранения | APPROX 4°C |
| растворимость | Chloroform (Soluble), DMSO (Sparingly), Ethyl Acetate (Sparingly), Methanol (Spa |
| форма | Crystalline Powder, Crystals and/or Chunks |
| цвет | Yellow or yellow-green to gray-beige |
| Растворимость в воде | insoluble |
| БРН | 1907918 |
| констант закона Генри | 5.53, 8.59, 13.0, 19.3, and 26.8(x 10-6 atm?m3/mol) at 4.1, 11.0, 18.0, 25.0, and 31.0 °C, respectively (Bamford et al., 1998) |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| Справочник по базе данных CAS | 206-44-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | 360UOL779Z |
| МАИР | 3 (Vol. Sup 7, 92) 2010 |
| Справочник по химии NIST | Fluoranthene(206-44-0) |
| Система регистрации веществ EPA | Fluoranthene (206-44-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,N,F,T | |||||||||
| Заявления о рисках | 22-36/37/38-40-67-65-50/53-38-11-39/23/24/25-23/24/25-52/53-50 | |||||||||
| Заявления о безопасности | 37/39-26-36/37-24/25-23-62-61-60-45-16-7 | |||||||||
| РИДАДР | UN 1593 6.1/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | LL4025000 | |||||||||
| кода HS | 29029090 | |||||||||
| Банк данных об опасных веществах | 206-44-0(Hazardous Substances Data) | |||||||||
| Токсичность | LC50 (24-h) for Daphnia magna 1,300 mg/L (LeBlanc, 1980), Cyprinodon variegatus >560 ppm (H Acute oral LD50 for rats 2,000 mg/kg (quoted, RTECS, 1985). | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Фторантен химические свойства, назначение, производство
Химические свойства
Fluoranthene is a polycyclic hydrocarbon and a colorless crystalline solid.Использование
Fluoranthene is a component of polynuclear aromatic hydrocarbons, also known as polycyclic aromatic hydrocarbons, and is usually bound to small particulate matter present in urban air, industrial and natural combustion emissions, and cigarette smoke.Определение
ChEBI: An ortho- and peri-fused polycyclic arene consisting of a naphthalene and benzene unit connected by a five-membered ring.Общее описание
Light yellow fine crystals.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Vigorous reactions, sometimes amounting to explosions, can result from the contact between aromatic hydrocarbons, such as Fluoranthene, and strong oxidizing agents. They can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction.Опасность
Questionable carcinogen.Угроза здоровью
ACUTE/CHRONIC HAZARDS: When heated to decomposition Fluoranthene emits acrid smoke and fumes.Пожароопасность
Flash point data for Fluoranthene are not available. Fluoranthene is probably combustible.Профиль безопасности
Poison by intravenous route. Moderately toxic by ingestion and skin contact. Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
Fluoranthene, a PAH, is produced from the pyrolytic processing of organic raw materials, such as coal and petroleum at high temperatures. It is also known to occur naturally as a product of plant biosynthesis. Fluoranthene is ubiquitous in the environment and has been detected in United States air; in foreign and domestic drink ing waters and in food-stuffs. It is also contained in ciga rette smoke. Individuals living in areas which are heavily industrialized; and in which large amounts of fossil fuels are burned, would be expected to have greatest exposure from ambient sources of fluoranthene. In addition, certain occupations e.g., coke oven workers, steelworkers, roofers, automobile mechanics) would also be expected to have elevated levels of exposure relative to the general popula tion. Exposure to fluoranthene will be considerably increased among tobacco smokers or those who are exposed to smokers in closed environments (i.e., indoors).Перевозки
UN1325 Flammable solids, organic, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid.UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Методы очистки
Fluoranthene (benzo[j,k]fluorene) M 202.3, m 110-111o, b 384o/760mm. Purify it by chromatography of CCl4 solutions on alumina, with *benzene as eluent. Crystallise it from EtOH, MeOH or *benzene. Also purify it by zone melting. [Gorman et al. J Am Chem Soc 107 4404 1985, Beilstein 5 I 344, 5 IV 2463.]Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Compound can react exo thermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel Crafts reaction.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must con form with EPA regulations governing storage, transportation, treatment, and waste disposal.Фторантен запасные части и сырье
Фторантен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-021-62885108 +8613917661608 |
China | 2068 | 57 | |
| +86-371-66670886 | China | 19902 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-13806087780 | China | 17365 | 58 |