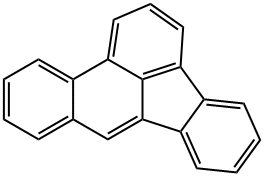
БЕНЗО(b)ФТОРАНТЭН
- английское имяBENZO(B)FLUORANTHENE
- CAS №205-99-2
- CBNumberCB5302581
- ФормулаC20H12
- мольный вес252.31
- EINECS205-911-9
- номер MDLMFCD00010582
- файл Mol205-99-2.mol
| Температура плавления | 163-165 °C(lit.) |
| Температура кипения | 481°C(lit.) |
| плотность | 1.1549 (estimate) |
| давление пара | 5 x 10-7 mmHg at 20 °C (U.S. EPA, 1982) |
| показатель преломления | 1.8390 (estimate) |
| Fp | -18 °C |
| температура хранения | 2-8°C |
| растворимость | Soluble in most solvents (U.S. EPA, 1985) |
| форма | solid |
| пка | >15 (Christensen et al., 1975) |
| цвет | White to Yellow to Green |
| Биологические источники | rabbit |
| Растворимость в воде | 1.2 μg/L at 25 °C (U.S. EPA, 1980a) |
| БРН | 1872553 |
| констант закона Генри | 2.47, 5.03, 11.74, 14.90, 20.53, and 36.52 at 10.0, 20.0, 35.0, 40.1, 45.0, and 55.0 °C, respectively (wetted-wall column, ten Hulscher et al., 1992) |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| Справочник по базе данных CAS | 205-99-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | FJO154KG1X |
| Предложение 65 Список | Benzo[b]fluoranthene |
| МАИР | 2B (Vol. 92) 2010 |
| Система регистрации веществ EPA | Benzo[b]fluoranthene (205-99-2) |
| UNSPSC Code | 41116107 |
| NACRES | NA.41 |
| Коды опасности | T,N,Xn,F,Xi | |||||||||
| Заявления о рисках | 45-50/53-67-65-38-11-36-39/23/24/25-23/24/25-66-52/53-20 | |||||||||
| Заявления о безопасности | 53-45-60-61-62-26-16-9-36/37-7-33-25 | |||||||||
| РИДАДР | UN 2811 6.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | CU1400000 | |||||||||
| кода HS | 2902.90.9000 | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 205-99-2(Hazardous Substances Data) | |||||||||
| Токсичность | LC50 (21-d) for Folsomia fimetaria >3,600 mg/kg (Sverdrup et al., 2002). | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H350:Может вызывать раковые заболевания.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P391:Ликвидировать просыпания/проливы/утечки.
P405:Хранить в недоступном для посторонних месте.
БЕНЗО(b)ФТОРАНТЭН химические свойства, назначение, производство
Химические свойства
Benzo(b)fluoranthene is a colorless, needleshaped solid.Физические свойства
Colorless to pale yellow to yellow-orange needles or crystals from benzene, toluene, ethylbenzene or acetic acid.Использование
Benz[e]acephenanthrylene is a polycyclic aromatic hydrocarbon that was detected in significant levels in airborne polluants, auto exhaust and tobacco and marijuana smoke.Определение
ChEBI: An ortho- and peri-fused polycyclic arene that consists of a benzene ring fused with a acephenanthrylene ring.Общее описание
Needles or yellow fluffy powder.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
BENZO(B)FLUORANTHENE can react with strong oxidizing agents. May react with electrophiles, peroxides, nitrogen oxides and sulfur oxidesОпасность
Confirmed carcinogen.Угроза здоровью
ACUTE/CHRONIC HAZARDS: When heated to decomposition, BENZO(B)FLUORANTHENE emits acrid smoke and irritating fumes.Пожароопасность
Flash point data for BENZO(B)FLUORANTHENE are not available; however, BENZO(B)FLUORANTHENE is probably combustible.Профиль безопасности
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
There is no commercial production of this compound. Benzo(b)fluoranthene is a chemical substance formed during the incomplete burning of fossil fuel, garbage, in cigarette smoke, or any organic matter and is Benzofluoranthene 399 found in smoke in general; it is carried into the air, where it condenses onto dust particles and is distributed into water and soil and on crops. B(b)F is a PAH and a component of coal tar pitch used in industry as a binder for electrodes. It is also a component of creosote, which is used to preserve wood. PAHs are also found in limited a mounts in bituminous materials and asphalt used for paving, roofing, and insulation. B(b)F has some use as a research chemical. It is available from some specialty chemical firms in low quantities (25 100 mg).Канцерогенность
Benzo[b]fluoranthenewas tested for carcinogenicity by dermal application in mice in multiple studies, intraperitoneal injection into mice in multiple studies, and intrapulmonary implantation into rats in one study. In all of these studies, benzo[b]-fluoranthene exhibited a significant carcinogenic activity.Экологическая судьба
Biological. Ye et al. (1996) investigated the ability of Sphingomonas paucimobilis strain U.S. EPA 505 (a soil bacterium capable of using fluoranthene as a sole source of carbon and energy) to degrade 4, 5, and 6-ringed aromatic hydrocarbons (10 ppm). After 16 h of incubation using a resting cell suspension, only 12.5% of benzo[b]fluoranthene had degraded. It was suggested that degradation occurred via ring cleavage resulting in the formation of polar metabolites and carbon dioxide.Soil. The reported half-lives for benzo[b]fluoranthene in a Kidman sandy loam and McLaurin sandy loam are 294 and 211 d, respectively (Park et al., 1990).
Photolytic. The atmospheric half-life was estimated to range from 1.43 to 14.3 h (Atkinson, 1987).
Chemical/Physical. Benzo[b]fluoranthene will not hydrolyze because it has no hydrolyzable functional group (Kollig, 1993).
Перевозки
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials, Technical Name Required.Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.Утилизация отходов
Residues and sorbent media may be packaged in 17H epoxy-lined drums and disposed of at an EPA-approved site. Destroy by permanganate oxidation, high-temperature incineration with scrubbing equipment, or microwave plasma treatment, if available. Confirm disposal procedures with responsible environmental engineer and regulatory officials.БЕНЗО(b)ФТОРАНТЭН запасные части и сырье
БЕНЗО(b)ФТОРАНТЭН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-0086-577-64498589 +86-15355981851 |
China | 14430 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| +86-27-87465837 +8618971612321 |
China | 9639 | 58 | |
| +86-61984905-1 +86-18016477331 |
China | 92 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 |