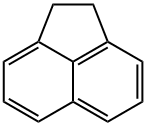
Аценафтен
- английское имяAcenaphthene
- CAS №83-32-9
- CBNumberCB7854448
- ФормулаC12H10
- мольный вес154.21
- EINECS201-469-6
- номер MDLMFCD00003807
- файл Mol83-32-9.mol
химическое свойство
| Температура плавления | 90-94 °C(lit.) |
| Температура кипения | 279 °C(lit.) |
| плотность | 1.06 |
| плотность пара | 5.32 (vs air) |
| давление пара | 10 mm Hg ( 131 °C) |
| показатель преломления | 1.6048 |
| Fp | 135 °C |
| температура хранения | Store below +30°C. |
| растворимость | chloroform: 50 mg/mL, clear |
| форма | crystalline |
| цвет | brown-beige |
| Растворимость в воде | 0.000347 g/100 mL |
| Мерк | 14,28 |
| БРН | 386081 |
| констант закона Генри | 3.47, 6.21, 10.8, 18.3, and 28.2 at 4.1, 11.0, 18.0, 25.0, and 31.0 °C, respectively (Bamford et al., 1998) |
| Диэлектрическая постоянная | 3.0 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| LogP | 4.04 at 30℃ and pH5-8 |
| Справочник по базе данных CAS | 83-32-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | V8UT1GAC5Y |
| МАИР | 3 (Vol. 92) 2010 |
| Справочник по химии NIST | Acenaphthene(83-32-9) |
| Система регистрации веществ EPA | Acenaphthene (83-32-9) |
| UNSPSC Code | 77101502 |
| NACRES | NA.24 |
больше
| Коды опасности | Xi,N,T,F,Xn | |||||||||
| Заявления о рисках | 36/37/38-50/53-39/23/24/25-23/24/25-11-52/53-67-65-38-51/53-20 | |||||||||
| Заявления о безопасности | 26-36/37/39-60-61-37/39-45-36/37-16-7-62-36-33-25-9 | |||||||||
| РИДАДР | UN 3077 9/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | AB1000000 | |||||||||
| Температура самовоспламенения | >450 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 9 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29029080 | |||||||||
| Банк данных об опасных веществах | 83-32-9(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: > 16000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Аценафтен химические свойства, назначение, производство
Описание
Acenaphthene is a tricyclic aromatic hydrocarbon and crystalline solid at ambient temperature. Acenaphthene does not dissolve in water but is soluble in many organic solvents. Acenaphthene is a component of crude oil and a product of combustion. Acenaphthene occurs in coal tar produced during the high-temperature carbonisation or coking of coal. It is used as a dye intermediate in the manufacture of some plastics and as an insecticide and fungicide. Acenaphthene is a component of crude oil and a product of combustion which may be produced and released to the environment during natural fires. Emissions from petroleum refining, coal tar distillation, coal combustion, and diesel-fuelled engines are major contributors of acenaphthene to the environment. Acenaphthene is an environmental pollutant and has been detected in cigarette smoke, automobile exhausts, and urban air; in effluents from petrochemical, pesticide, and wood preservative industries; and in soils, groundwater, and surface waters at hazardous waste sites. This compound is one among a number of polycyclic aromatic hydrocarbons (PAHs) on U.S. EPA’s (Environmental Protection Agency) priority pollutant list.Химические свойства
Acenaphthene is a tricyclic aromatic hydrocarbon, crystalline solid at ambient tempera- ture. Acenaphthene does not dissolve in water, but is soluble in many organic solvents. Acenaphthene occurs in coal tar produced during high temperature carbonization or cok- ing of coal. It is used as a dye intermediate in the manufacture of some plastics and as an insecticide and fungicide. Acenaphthene is a component of crude oil and a product of combustion that may be produced and released into the environment during natural fi res. Emissions from petroleum refi ning, coal tar distillation, coal combustion, and diesel- fueled engines are major contributors of acenaphthene to the environment. Acenaphthene is an environmental pollutant and has been detected in cigarette smoke, automobile exhausts, and urban air; in effl uents from petrochemical, pesticide, and wood preservative industries; and in soils, groundwater, and surface waters at hazardous waste sites. This compound is one of a number of polycyclic aromatic hydrocarbons on the US EPA’s prior- ity pollutant list.Физические свойства
White crystalline solid or orthorhombic bipyramidal needles from alcohol. Coal tar-like odor. The lowest odor threshold concentration in water that may result in rejection of contaminated water ranged from 0.02 to 0.22 ppm (Lillard and Powers, 1975). In Wisconsin, the taste and odor threshold concentration in water that is nontoxic to humans is 20 μg/L (ATSDR, 1995).Использование
Acenaphthene occurs in petroleum bottoms and is used as a dye intermediate, insecticide, and fungicide and in manufacturing plastics.Определение
acenaphthene: A colourless crystallinearomatic compound, C12H10;m.p. 95°C; b.p. 278°C. It is an intermediatein the production of somedyes.Общее описание
White needles. Melting point 93.6°C. Soluble in hot alcohol. Denser than water and insoluble in water. Hence sinks in water. May irritate skin and mucous membranes. Emits acrid smoke and irritating fumes when heated to decomposition. Derived from coal tar and used to make dyes, pharmaceuticals, insecticides, fungicides, and plastics.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Acenaphthene is incompatible with strong oxidizing agents. Incompatible with ozone and chlorinating agents. Forms crystalline complexes with desoxycholic acid .Угроза здоровью
Exposures to acenaphthene cause poisoning and include symptoms such as irritation to the skin, eyes, mucous membranes, and upper respiratory tract. Studies on labora- tory animals orally exposed to acenaphthene showed loss of body weight, peripheral blood changes (unspecifi ed), increased aminotransferase levels in blood serum, and mild morphological damage to the liver and kidneys. In chronic exposures, acenaph- thene is known to cause damage to the kidneys and liver. Acenaphthene is irritating to the skin and mucous membranes of humans and animals. Oral exposure of rats to ace- naphthene for 32 days produced peripheral blood changes, mild liver and kidney dam- age, and pulmonary effects. However, detailed studies with acenaphthene in humans are limited.Пожароопасность
Flash point data for Acenaphthene are not available. Acenaphthene is probably combustible.Профиль безопасности
Moderately toxic by intraperitonealroute. Mutation data reported.Incompatible with strongoxidizing agents, ozone, chlorinating agents. When heatedto decomposition it emits acrid smoke and irritating vapors.Возможный контакт
Acenaphthene occurs naturally in coal tar and in coal tar produced during the high-temperature carbonization or coking of coal; coal tar distilling; petroleum processing; shale oil processing. It is used as an intermediate for dyes, fungicides, insecticides, herbicides, pharmaceuticals, plant growth hormones; 1,8 naphthalic acid; in the manufacture of some plastics; and has been detected in cigarette smoke and gasoline exhaust condensates; a constituent of coal tar creosote, asphalt, and diesel fuel. It has been used as an polyploidy agent.Перевозки
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Методы очистки
It has also been purified by chromatography from CCl4 on alumina with *benzene as eluent [McLaughlin & Zainal J Chem Soc 2485 1960]. [Beilstein 5 IV 1834.]Несовместимости
Ozone and strong oxidizing agents, including perchlorates, chlorine, fluorine, and bromineУтилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Incineration or permanganate oxidationАценафтен запасные части и сырье
сырьё
запасной предмет
- 1,4,5,8-Naphthalenetetracarboxylic acid
- 1-АМИНО-8-НАФТОЕВАЯ КИСЛОТА
- Аценафталин
- 1,8-НАФТАЛЕВАЯ КИСЛОТА
- fluorescent whitening agent EFR
- аценафтенхинон
- 1,8-нафталевый ангидрид
- 4,5-дихлорнафталин-1,8-дикарбоновой ангидрид
- 5-НИТРОАЦЕНАФТЕН
- 4-Bromo-1,8-naphthalic anhydride
1of4
Аценафтен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +8613367258412 | China | 10319 | 58 |