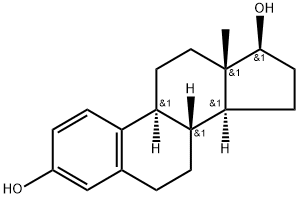
Эстрадиол
- английское имяβ-Estradiol
- CAS №50-28-2
- CBNumberCB2200244
- ФормулаC18H24O2
- мольный вес272.39
- EINECS200-023-8
- номер MDLMFCD01074033
- файл Mol50-28-2.mol
| Температура плавления | 178-179 °C(lit.) |
| Температура кипения | 355.44°C (rough estimate) |
| альфа | D25 +76 to +83° (dioxane) |
| плотность | 1.0708 (rough estimate) |
| показатель преломления | 80.4 ° (C=1, Dioxane) |
| Fp | 2℃ |
| температура хранения | room temp |
| растворимость | Practically insoluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent), slightly soluble in methylene chloride. |
| форма | powder |
| пка | pKa 10.71±0.02(H2O(0.1% p-dioxane) t=25±0.1 I=0.03(KCl))(Approximate) |
| цвет | White to off-white |
| Биологические источники | synthetic (organic) |
| Растворимость в воде | Soluble in dimethyl sulfoxide, ethanol , water, phosphate buffer saline, dimethyl formamide, acetone, dioxane and alkali hydroxides. Slightly soluble in vegetable oils. |
| Мерк | 14,3703 |
| БРН | 1914275 |
| BCS Class | 1 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | VOXZDWNPVJITMN-ZBRFXRBCSA-N |
| Справочник по базе данных CAS | 50-28-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3-6 |
| Словарь онкологических терминов NCI | estradiol |
| FDA UNII | 4TI98Z838E |
| Словарь наркотиков NCI | ESTRING |
| Код УВД | G03CA03,G03CA53 |
| Справочник по химии NIST | Estra-1,3,5(10)-triene-3,17beta-diol(50-28-2) |
| Система регистрации веществ EPA | Estradiol (50-28-2) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Коды опасности | T,Xn,F | |||||||||
| Заявления о рисках | 60-61-45-63-64-40-36-20/21/22-11-48 | |||||||||
| Заявления о безопасности | 53-22-36/37/39-45-36/37-26-16-36-20 | |||||||||
| РИДАДР | 2811 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | KG2975000 | |||||||||
| F | 8-10 | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29372390 | |||||||||
| Банк данных об опасных веществах | 50-28-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 subcutaneous in rat: > 300mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H362:Может причинить вред детям, находящимся на грудном вскармливании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H360FD:Может отрицательно повлиять на способность к деторождению. Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P263:Избегать контакта в период беременности и грудного вскармливания.
P264:После работы тщательно вымыть кожу.
P273:Избегать попадания в окружающую среду.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Эстрадиол химические свойства, назначение, производство
Химические свойства
White or almost white, crystalline powder or colourless crystals.Использование
17β-Estradiol is the major estrogen secreted by the premenopausal ovary.This compound is a contaminant of emerging concern (CECs). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants.Определение
ChEBI: The 17beta-isomer of estradiol.прикладной
β-Estradiol has been used:for the in vitro maturation of bovine cumulus-oocyte complexes (COCs)
as a supplement in in vitro maturation medium (IVM), which is used as a control medium
in estrogen-induction assay
Приобретенная устойчивость
Estradiol is the most potent endogenous estrogen, exhibiting high affinity for the ER and high potency when administered parenterally. When administered orally, estradiol is promptly conjugated in the intestine and oxidatively metabolized by the liver, resulting in its low oral bioavailability and therapeutic effectiveness.Общее описание
Estradiol, estra-1,3,5(10)-triene-3,17β-diol, is the most activeof the natural steroid estrogens. Although its 17β-OHgroup is vulnerable to bacterial and enzymatic oxidation toestrone, it can be temporarily protected as anester at C3 or C17, or permanently protected by adding a17α-alkyl group (e.g., 17α-ethinyl estradiol, the most commonlyused estrogen in oral contraceptives). The increasedoil solubility of the 17β-esters (relative to estradiol) permitsthe esters to remain in oil at the IM injection site for extendedperiods. These derivatives illustrate the principles of steroidmodification. Transdermal estradiolproducts avoid first-pass metabolism, allowing estradiol tobe as effective as oral estrogens for treating menopausalsymptoms. A new transdermal spray, Evamist, was approvedin 2007. Estradiol itself is typically not very effective orallybecause of rapid metabolism, but an oral formulation of micronizedestradiol that allows more rapid absorption of thedrug is available (Estrace). In addition to the oral and transdermalproducts, estradiol is also available in gel, cream, andvaginal ring formulations. The commercially available estradiolesters are the following:Estradiol 3-acetate, USP (oral; vaginal ring)
Estradiol 17-valerate, USP (IM injection)
Estradiol 17-cypionate, USP (IM injection).
Опасность
A carcinogen (OSHA).Биологическая активность
Endogenous estrogen receptor (ER) agonist (K i values are 0.12 and 0.13 nM for ER α and ER β respectively). Also high affinity ligand at membrane estrogen GPR30 receptors.Контактные аллергены
Natural estradiol, used in transdermal systems for hormonal substitution, can induce allergic contact dermatitis, with the risk of systemic contact dermatitis after oral reintroduction.Механизм действия
The most potent naturally occurring estrogen in mammals. It is synthesized primarily in the ovary, and also in the testis, adrenal gland and placenta, and to a limited extent by peripheral tissues (e.g., liver, fat, and skeletal muscle) from androstenedione and testosterone. It is responsible for the development of secondary sex characteristics in the female at puberty (i.e., growth and development of the vagina, uterus and fallopian tubes, enlargement of the breasts, and growth and maturation of long bones).Профиль безопасности
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. A promoter. Human reproductive effects by ingestion: ferthty effects. Experimental reproductive effects. Human mutation data reported. A steroid hormone much used in medicine. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
The working environment may be contaminated during sex hormone manufacture, especially during the extraction and purification of natural steroid hormones; grinding of raw materials; handling of powdered products and recrystallization. Airborne particles of sex hormones may be absorbed through the skin, ingested or inhaled. Enteric absorption results in quick inactivation of sex hormones in the liver. The rate of inactivation is decreased for the oral, alkylated steroid hormones (methyl testosterone, anabolic steroids, etc.). Sex hormones may accumulate and reach relatively high levels even if their absorption is intermittent. Consequently, repeated absorption of small amounts may be detrimental to health. Intoxication by sex hormones may occur in almost all the exposed workers if preventive measures are not taken. The effect in the industrial sector is more successful than the agricultural one (chemical caponizing of cockerels by stilbestrol implants and incorporation of estrogens in feed for body weight gain promotion in beef cattle), where measures taken are summary and the number of cases of intoxication is consequently biggerПеревозки
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materialsМетоды очистки
17-Estradiol (previously known as -estradiol) is purified by chromatography on SiO2 (toluene/EtOAc 4:1) and recrystallised from CHCl3/hexane or 80% EtOH. It is stable in air, is insoluble in H2O, and is precipitated by digitonin. The UV has max at 225 and 280 nm. The diacetate [3434-88-6] has m 97-98o and forms leaflets from aqueous EtOH. The 3-benzoate crystallises from aqueous MeOH withm 193o and [] D 25 +58o to 63o (c 1, dioxane). [Meischer & Scholz Helv Chim Acta 20 263, 1237 1937, Biochem J 32 1273 1938, Oppolzer & Roberts Helv Chim Acta 63 1703 1980, Inhoffen & Zühlsdorff Chem Ber 7 4 1914 1941, Beilstein 6 IV 6611.]Эстрадиол запасные части и сырье
сырьё
1of3
запасной предмет
Эстрадиол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8617786394783 | China | 300 | 58 | ||
| +undefined-27-86652399 +undefined13627115097 |
China | 942 | 58 | ||
| +86-15271838296; +8615271838296 |
China | 1512 | 58 | ||
| +86-0533-2066820 +8618369939125 |
China | 1009 | 58 | ||
| +86-027-59207850 | China | 5972 | 58 | ||
| +8617709210191 | China | 882 | 58 | ||
| +86-18031140164 +86-19933155420 |
China | 100 | 58 | ||
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | ||
| +86-86-13583358881 +8618560316533 |
China | 3094 | 58 | ||
| +8615387054039 | China | 427 | 58 |