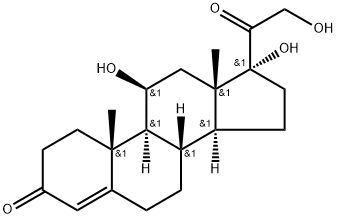
Гидрокортизон
- английское имяHydrocortisone
- CAS №50-23-7
- CBNumberCB9756715
- ФормулаC21H30O5
- мольный вес362.47
- EINECS200-020-1
- номер MDLMFCD00011654
- файл Mol50-23-7.mol
| Температура плавления | 211-214 °C(lit.) |
| альфа | 166 º (c=1, C2H5OH 25 ºC) |
| Температура кипения | 414.06°C (rough estimate) |
| плотность | 1.0812 (rough estimate) |
| показатель преломления | 1.6120 (estimate) |
| Fp | 220°C |
| температура хранения | -20°C |
| растворимость | H2O: 100 mg/mL |
| форма | powder |
| цвет | White |
| РН | 5.0- 7.0 |
| Биологические источники | non-animal source |
| Растворимость в воде | 319.7mg/L(25 ºC) |
| Разложение | 220 ºC |
| Мерк | 14,4787 |
| БРН | 1354819 |
| Стабильность | Stable, but may be light sensitive. Incompatible with strong oxidizing agents. |
| LogP | 1.610 |
| Справочник по базе данных CAS | 50-23-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| Словарь онкологических терминов NCI | cortisol; hydrocortisone |
| FDA UNII | WI4X0X7BPJ |
| Словарь наркотиков NCI | Aeroseb-HC |
| Код УВД | A01AC03,A07EA02,C05AA01,D07AA02,D07XA01,H02AB09,R01AD60,S01BA02,S01CB03,S02BA01 |
| Справочник по химии NIST | Hydrocortisone(50-23-7) |
| Система регистрации веществ EPA | Hydrocortisone (50-23-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 62-63 | |||||||||
| Заявления о безопасности | 36/37 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | GM8925000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29372100 | |||||||||
| Банк данных об опасных веществах | 50-23-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 subcutaneous in mouse: > 500mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H360Df:Может отрицательно повлиять на неродившегося ребенка. Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Гидрокортизон химические свойства, назначение, производство
Химические свойства
crystalline white powderИспользование
Cortisol, or Hydrocortisone, is a steroid hormone, more specifically a glucocorticoid, produced by the zona fasciculata of the adrenal gland. Cortisol is released in response to stress and a low level of blood glucocorticoids. Its primary functions are to increase blood sugar through gluconeogenesis; suppress the immune system; and aid in fat, protein and carbohydrate metabolism.Определение
ChEBI: A C21-steroid that is pregn-4-ene substituted by oxo groups at positions 3 and 20 and hydroxy groups at positions 11, 17 and 21. Cortisol is a corticosteroid hormone or glucocorticoid produced by zona fasciculata of the adrenal cortex, which is a part of the adrenal gland. It is usually referred to as the "stress hormone" as it is involved in response to stress and anxiety, controlled by corticotropin-releasing hormone (CRH). It increases blood pressure and blood sugar, and reduces immun responsesПоказания
Hydrocortisone (Cortizone, Cortaid, Anusol-HC, Hytone, LactiCare-HC, Sarnol HC, Penecort, Texacort, and many other branded products) may be purchased as a generic drug.Общее описание
Hydrocortisone, 11β,17,21-trihydroxypregn-4-ene-3,20-dione, is the primary natural GCin humans. Despite the large number of synthetic GCs, hydrocortisone,its esters, and its salts remain a mainstay ofmodern adrenocortical steroid therapy and the standard forcomparison of all other GCs and MCs . It isused for all the indications mentioned previously.Угроза здоровью
Cortisol Increases (1) protein catabolism (excepting liver) gluconeogenesis; (2) carbohydrate anabolism (liver); (3) blood sugar; (4) glucose absorption; (5) brain excitation; (6) spread of infections; (7)urinary glucose and nitrogen; (8) stress tolerance; (9) lactation; (10) water diuresis.Regulates general adaptation syndrome, water balance, blood pressure, and hormone release.
Decreases (1) fat anabolism; (2) growth rate; (3) inflammation; (4) eosinophils; (5) lymphocytes; (6) antigen sensitivity; (7) respiratory quotient; (8) ketosis; (9) wound healing; (10) skin pigmentation; (11)RBC hemolysis.
Контактные аллергены
Hydrocortisone is the principal glucocorticoid hor- mone produced by the adrenal cortex and is used topi- cally or systemically. It belongs to the allergenic A group. Marker of allergy is tixocortol pivalate.Механизм действия
Hydrocortisone exhibits anti-shock, anti-allergy, and anti-inflammatory action. It raises sugar content in the blood, increases potassium secretion, and lowers sodium excretion from the body. It exhibits anti-metabolic action and reduces histamine synthesis in the body.Клиническое использование
Hydrocortisone is endogenous, and it has both glucocorticoid and mineralocorticoid activity. It is the fundamental structure by which the glucocorticoid and mineralocorticoid activities of all other corticosteroids are judged. Functional groups that are essential for both mineralocorticoid and glucocorticoid activity include the pregnane skeleton with an all-trans backbone, the ring A-en-one system (?4 -3-one ring A) and the 17β-ketol side chain (C-20-keto-C-21-hydroxy). The glucocorticoid activity is enhanced by the C-11 and C-17 hydroxyl groups. Hydrocortisone can be used to treat severe asthmatic attacks that do not respond to conventional treatment. It is available as various ester forms.Профиль безопасности
Poison byМетоды очистки
Recrystallise hydrocortisone from EtOH or isoPrOH. It is bitter tasting and has UV with max at 242 nm (log 4.20). Its solubility at 25o is: H2O (0.28%), EtOH (1.5%), MeOH (0.62%), Me2CO (0.93%), CHCl3 (0.16%), propylene glycol (1.3%) and Et2O (0.35%). It gives an intense green colour with conc H2SO4. [Wendler et al. J Am Chem Soc 72 5793 1950, Beilstein 8 IV 3422.]Гидрокортизон запасные части и сырье
сырьё
1of2
запасной предмет
- 6,9-Difluoropregn-4-ene-11,17,21-triol-3,20-dione17,21-diacetate
- 9-Bromo-11,17,21-trihydroxypregn-4-ene-3,20-dione17,21-diacetate
- 9-Bromo-11,17,21-trihydroxypregn-4-ene-3,20-dione 21-acetate
- 9-Fluoropregna-1,4-diene-11,17,21-triol-3,20-dione17,21-diacetate
- 6,9-Difluoro-11,16,17,21-tetrahydroxypregna-1,4-diene-3,20-dione 21-acetate
- 6-Fluoro-17,21-dihydroxypregna-4,9(11)-diene-3,20-dione 17,21-diacetate
- 11alpha,17,21-trihydroxypregn-4-ene-3,20-dione 21-acetate
- 9,11-Epoxy-6-fluoro-17,21-dihydroxypregn-4-ene-3,20-dione-17,21-diacetate
- 6alpha,9-difluoro-11beta,21-dihydroxypregna-1,4,16-triene-3,20-dione 21-acetate
- Преднизолон
- 9,11-Epoxypregn-4-ene-17,21-diol-3,20-dione 21-acetate
- 17,21-Диацетилокси-9,11-эпоксипрегн-4-ен-3,20-дион
- Pregna-4,9(11)-diene-17,21-diol-3,20-dione17,21-diacetate
- АНЕКОРТАВА АЦЕТАТ (200 мг) F0E2980.997MG / MG (AI)
- Deprodone propionate
1of5
Гидрокортизон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8617798174412 | China | 2097 | 58 | |
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | |
| +8615713292910 | China | 341 | 58 | |
| +86-18239973690 +86-18239973690 |
China | 311 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| 010-60279497 | CHINA | 1803 | 55 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +8615102730682 | CHINA | 566 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 |