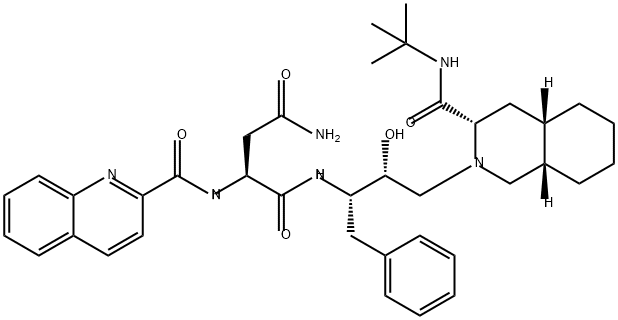
Саквинавир
- английское имяSaquinavir
- CAS №127779-20-8
- CBNumberCB2161048
- ФормулаC38H50N6O5
- мольный вес670.84
- EINECS1806241-263-5
- номер MDLMFCD00866925
- файл Mol127779-20-8.mol
| альфа | D20 -55.9° (c = 0.5 in methanol) |
| Температура кипения | 1015.0±65.0 °C(Predicted) |
| плотность | 1.211±0.06 g/cm3(Predicted) |
| температура хранения | Sealed in dry,Store in freezer, under -20°C |
| растворимость | DMSO : 100 mg/mL (149.07 mM; Need ultrasonic) |
| форма | Powder |
| пка | 11.05±0.46(Predicted) |
| цвет | White to off-white |
| Растворимость в воде | 35.8mg/L(25 ºC) |
| BCS Class | 4 |
| FDA 21 CFR | 556.286 |
| Справочник по базе данных CAS | 127779-20-8(CAS DataBase Reference) |
| FDA UNII | L3JE09KZ2F |
| Код УВД | J05AE01 |
| Банк данных об опасных веществах | 127779-20-8(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338+P310:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз. Немедленно обратиться за медицинской помощью.
P363:Перед повторным использованием выстирать загрязненную одежду.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Саквинавир химические свойства, назначение, производство
Описание
Saquinavir mesylate, the first HIV protease inhibitor to reach the market, was launched in the U.S.A.. It is indicated for use in combination with approved nucleoside analogs for the treatment of advanced HIV infection. Saquinavir, a transition state analog of Phe-Pro, is a very potent and competitive inhibitor of HIV-1 and HIV-2 proteases with high specificity. Saquinavir inhibits the last stage in the replication process of HIV and prevents virion maturation in both acute and chronically infected cells. Combination of saquinavir with the nucleoside analogs such as zidovudine (AZT) or/and zalcitabine which inhibit the enzyme reverse transcriptase and target at an earlier stage in the HIV replication process, shows a greater than additive effect in increase in CD4 cell counts and reduction in viral load, with the combination delaying the onset of resistance to either drug alone. Saquinavir is well tolerated alone and in combination with A n .Использование
Antiviral (HIV protease inhibitor).Определение
ChEBI: An aspartic acid derivative obtained by formal condensation of the primary amino group of (2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarbamoyl)octahydroisoquinolin- (1H)-yl]-3-hydroxy-1-phenylbutan-2-ylamine with the carboxy group of N2(-quinolin-2-ylcarbonyl)-L-asparagine. An inhibitor of HIV-1 protease.Показания
Saquinavir is a potent inhibitor of HIV-1 and HIV-2 protease. Fortovase, a soft gel preparation of saquinavir, has largely replaced saquinavir mesylate capsules (Invirase) because it has improved bioavailability. Saquinavir is usually well tolerated and most frequently produces mild gastrointestinal side effects.Приобретенная устойчивость
Resistance is associated with an amino acid substitution at position 48 in the HIV protease (G48V). An L90M mutation also confers resistance, as it does for most protease inhibitors. Saquinavir-resistant isolates from patients on long-term therapy often show cross-resistance to other protease inhibitors.Общее описание
Saquinavir (Invirase) is well tolerated following oral administration.Absorption of saquinavir is poor but is increasedwith a fatty meal. The drug does not distribute intothe CSF, and it is approximately 98% bound to plasma proteins.Saquinavir is extensively metabolized by the firstpasseffect. Bioavailability is 4% from a hard capsule and12% to 15% from a soft capsule. Saquinavir lowers p24antigen levels in HIV-infected patients, elevates CD4+counts, and exerts a synergistic antiviral effect when combinedwith RT inhibitors such as AZT and ddC.Although HIV-1 resistance to saquinavir and other HIVPIs occurs in vivo, it is believed to be less stringent andless frequent than resistance to the RT inhibitors.Nevertheless,cross-resistance between different HIV PIsappears to be common and additive,suggesting thatusing combinations of inhibitors from this class would notconstitute rational prescribing. The drug should be used incombination with at least two other antiretroviral drugs tominimize resistance. Dosage forms are Invirase (hard capsule)and Fortovase (soft capsule).Фармацевтические приложения
A peptidomimetic protease inhibitor formulated as the mesylate for oral use.Фармакокине?тика
Oral absorption: c. 4%Cmax 1200 mg thrice daily: c. 1–2.2 mg/L
Cmin 1200 mg thrice daily: c. 0.1–0.22 mg/L
Plasma half-life: c. 7–12 h
Volume of distribution: c. 700 L
Plasma protein binding: c. 98%
Absorption and distribution
It is poorly absorbed and penetrates poorly into the CNS. The semen:plasma ratio is 0.04. It is not known if it is distributed into human breast milk.
Metabolism and excretion
It is metabolized via CYP3A4, principally to mono- and dihydroxylated derivatives. Around 88% of the dose is excreted in feces and 1% in urine. Caution should be exercised in severe renal impairment and moderate hepatic impairment; use in decompensated hepatic impairment is contraindicated.
Клиническое использование
Treatment of HIV infection (in combination with other antiretroviral drugs)Побочные эффекты
The most frequently reported adverse effects include abdominal discomfort, diarrhea and nausea. Ritonavir-boosted saquinavir is associated with a dyslipidemic profile characteristic of those treated with a boosted protease inhibitor requiring 200 mg of the ritonavir ‘booster’.Саквинавир запасные части и сырье
Саквинавир поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| 86-571-88216897,88216896 13588875226 |
CHINA | 6312 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| 0917-3909592 13892490616 |
China | 9310 | 58 | |
| +86-029-86333380 18829239519 |
China | 899 | 58 | |
| +86-0571-81612335 +8613336028439 |
China | 19908 | 58 | |
| +1-708-310-1919 +1-13798911105 |
United States | 6391 | 58 |