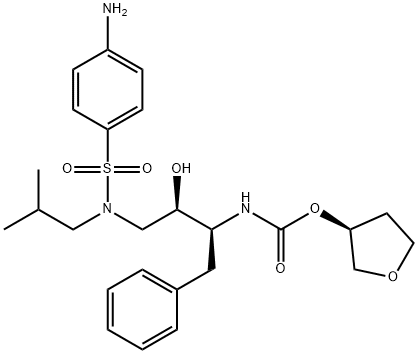
Ампренавир
- английское имяAMPRENAVIR
- CAS №161814-49-9
- CBNumberCB4186139
- ФормулаC25H35N3O6S
- мольный вес505.63
- EINECS827-179-5
- номер MDLMFCD00934214
- файл Mol161814-49-9.mol
| Температура плавления | 72-74°C |
| плотность | 1.30±0.1 g/cm3(Predicted) |
| температура хранения | -20°C |
| растворимость | DMSO: soluble20mg/mL, clear |
| форма | powder |
| пка | 11.54±0.46(Predicted) |
| цвет | white to beige |
| оптическая активность | [α]/D +8 to +12°, c = 0.5 in methanol |
| BCS Class | 2 |
| ИнЧИКей | YMARZQAQMVYCKC-OEMFJLHTSA-N |
| FDA UNII | 5S0W860XNR |
| Код УВД | J05AE05 |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
| РИДАДР | 3077 |
| WGK Германия | 3 |
| кода HS | 29350090 |
| Банк данных об опасных веществах | 161814-49-9(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
H332:Вредно при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Ампренавир химические свойства, назначение, производство
Описание
Amprenavir was launched as Agenerase in the US for the treatment of AIDS patients in combination with approved agent antiretroviral nucleoside analogs. It is the fifth non-peptidic inhibitor of HIV-1 protease to be marketed in this indication after the last approved Neflinavir. Amprenavir, designed via a structure-based process, is the smallest molecule in the 《navir》 class and exhibits a reduced peptidic character. An improved process for preparation comprising four steps from a (1S, 2R)-2-hydroxy-3-aminopropylcarbamate has been developed. Amprenavir is a potent inhibitor of HIV-1 aspartyl protease (Ki = 0.6nM), an enzyme required by the virus to cleave pro-form polyproteins to structural proteins during the last stage in the replication process. The compound displays good oral bioavailability in humans and penetrates the CNS, which is an important advantage in long-term treatment. Its plasma half-life is approximately 10h. Treatment with Amprenavir in combination with nucleoside analog reverse transcriptase inhibitors considerably decreases viral load and restores CD4+ T-cell counts in patients with HIV infection.Химические свойства
Off-White to Pale YellowИспользование
A selective HIV protease inhibitor. An analogue of RitonavirПоказания
Amprenavir (Agenerase) is administered twice daily, providing the patient with an advantage over other protease inhibitors that must be taken more frequently (e.g., indinavir, saquinavir). Common side effects of am-prenavir include nausea, vomiting, diarrhea, and perioral paraesthesias. Rash occurs in approximately 20 to 30% of patients and can be mild or severe (Stevens- Johnson syndrome).Приобретенная устойчивость
Mutations at position 50, 76 and 84 of the protease enzyme gene are associated with significantly reduced susceptibility.Общее описание
Amprenavir is a second-generation drug derived from hydroxyethylamine sulfonamide.Фармацевтические приложения
A synthetic compound formulated as the calcium salt of the oral prodrug fosamprenavir.Фармакокине?тика
Oral absorption: Not known/availableCmax 700 mg + ritonavir 100 mg:c. 6.08 mg/L
twice daily
Cmin 700 mg + ritonavir 100 mg:c. 2.12 mg/L
twice daily
Plasma half-life: c. 7.7 h
Volume of distribution: c. 430 L
Plasma protein binding: c. 90%
Absorption
Fosamprenavir is rapidly and almost completely hydrolyzed to amprenavir and inorganic phosphate by cellular phosphatases in the gut epithelium as it is absorbed. Absolute bioavailability has not been established. It can be taken without regard to food.
Distribution
It penetrates moderately well into the CNS. The semen:plasma ratio is negligible. It is not known if it is distributed into breast milk.
Metabolism and excretion
It is extensively metabolized by the cytochrome P450 (CYP) 3A4 enzyme system. Two major metabolites have been identified that appear to result from the oxidation of the tetrahydrofuran and aniline moieties. Around 14% of a dose is eliminated in the urine and 75% in feces, <3% as unchanged drug. Metabolites account for >90% of administered drug found in fecal samples. It should be used with caution and at reduced doses in adults with mild or moderate hepatic impairment; it is contraindicated in patients with severe hepatic impairment.
Клиническое использование
Treatment of HIV infection (in combination with other antiretroviral drugs)Побочные эффекты
The most common adverse events in patients receiving boosted fosamprenavir were diarrhea, nausea, headache, fatigue, vomiting and rash. Ritonavir-boosted fosamprenavir is associated with a dyslipidemia profile characteristic of those treated with other protease inhibitors boosted with 200 mg of ritonavir.Ампренавир запасные части и сырье
Ампренавир поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| 0086-13720134139 | CHINA | 965 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 8485655694 | United States | 63687 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| 86-571-88216897,88216896 13588875226 |
CHINA | 6312 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| 0917-3909592 13892490616 |
China | 9310 | 58 |