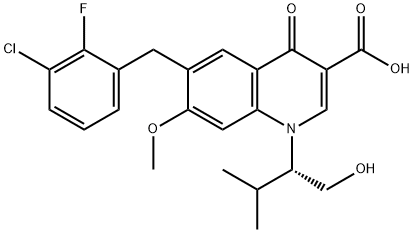
Элвитегравир
- английское имяElvitegravir
- CAS №697761-98-1
- CBNumberCB02464875
- ФормулаC23H23ClFNO5
- мольный вес447.88
- EINECS1592732-453-0
- номер MDLMFCD11846134
- файл Mol697761-98-1.mol
химическое свойство
| Температура плавления | 93-96°C |
| Температура кипения | 623.6±55.0 °C(Predicted) |
| плотность | 1.357±0.06 g/cm3(Predicted) |
| температура хранения | Refrigerator |
| растворимость | DMSO (Slightly), Methanol (Slightly) |
| пка | 0.44±0.20(Predicted) |
| форма | Solid |
| цвет | Off-White to Pale Yellow |
| оптическая активность | [α]/D -26 to -34°, c =0.5 in methanol |
| ИнЧИКей | JUZYLCPPVHEVSV-LJQANCHMSA-N |
| SMILES | N1([C@H](CO)C(C)C)C2=C(C=C(CC3=CC=CC(Cl)=C3F)C(OC)=C2)C(=O)C(C(O)=O)=C1 |
| Справочник по базе данных CAS | 697761-98-1 |
| FDA UNII | 4GDQ854U53 |
| Код УВД | J05AJ02 |
| UNSPSC Code | 12352200 |
| NACRES | NA.25 |
Элвитегравир химические свойства, назначение, производство
Описание
In November 2013, the European Medicines Agency (EMA) approved elvitegravir (also known as GS 9137 and JTK 303) as a single agent to be used as part of an antiviral regimen that includes a ritonavir-boosted protease inhibitor for the treatment of HIV-1 in adults without mutations indicative of elvitegravir resistance. Elvitegravir is the second of three marketed HIV integrase strand transfer inhibitors (INSTIs) including raltegravir and dolutegravir (this volume of ARMC). Elvitegravir was discovered by modification of a literature naphthyridine HIV integrase inhibitor in which the naphthyridine core served as a bioisostere for the diketo acid moiety in an original series. Serendipitously, a 4-quinolone-3-carboxylic acid precursor en route to the desired bioisosteric glyoxylic acid demonstrated modest integrase inhibition (IC50=1600 nM). Further derivatization led to elvitegravir with enhanced inhibition of integrase strand transfer (IC50=7.2 nM) and significant antiviral activity (EC50=0.9 nM). Elvitegravir was prepared in seven synthetic steps from 2,4-difluoro-5-iodobenzoic acid. The corresponding acid chloride was coupled to ethyl 3-(dimethylamino) acrylate and further substituted with S-valinol. Base promoted cyclization afforded the quinolone which was protected as silyl ether. Negishi coupling installed the 2-fluoro-3-chlorobenzyl moiety. Subsequent hydrolysis and methoxylation afforded elvitegravir.Химические свойства
Off-White to Pale Yellow SolidИспользование
A novel inhibitor of human immunodeficiency virus type 1 integrase.Определение
ChEBI: A quinolinemonocarboxylic acid that is 7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid substited at position 1 by a 1-hydroxy-3-methylbutan-2-yl group and at position 6 by a 3-chloro-2-fluorobenzyl group (the S-enantiomer). It is us d in combination therapy for the treatment of HIV-1 infection.Элвитегравир поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| 010-60279497 | CHINA | 1803 | 55 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 0086-13720134139 | CHINA | 965 | 58 | |
| 18627774460 | CHINA | 742 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +1-631-485-4226 | United States | 19553 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 |