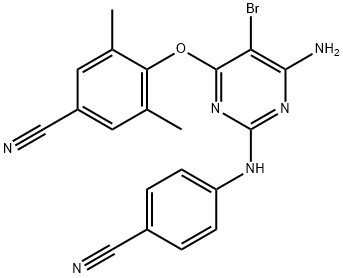
Этравирин
- английское имяEtravirine
- CAS №269055-15-4
- CBNumberCB51509336
- ФормулаC20H15BrN6O
- мольный вес435.28
- EINECS682-331-6
- номер MDLMFCD09837879
- файл Mol269055-15-4.mol
химическое свойство
| Температура плавления | .265°C (dec.) |
| Температура кипения | 637.4±65.0 °C(Predicted) |
| плотность | 1.439 g/cm3 |
| температура хранения | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| растворимость | DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) |
| форма | Solid |
| пка | 1.23±0.10(Predicted) |
| цвет | White to Off-White |
| FDA UNII | 0C50HW4FO1 |
| Код УВД | J05AG04 |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Этравирин химические свойства, назначение, производство
Описание
Etravirine is a second-generation NNRTI. It is indicated for use in combination with other antiretroviral agents for treating HIV-1 infection in treatment-experienced adult patients who have evidence of viral replication and HIV-1 strains resistant to the currently available NNRTIs and other antiretroviral agents. The NNRTIs, along with nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs/NtRTIs), are important components of the combination regimens currently used to treat HIV-1 infection. The NRTIs/NtRTIs act by competing with the natural nucleotide substrates of reverse transcriptase for incorporation into viral DNA and subsequent chain termination. By contrast, the NNRTIs bind to an allosteric site of the enzyme and disrupt the DNA polymerase function by inducing conformational changes to the catalytic site. The allosteric binding nature of NNRTIs generally results in improved safety profile since there is no known human homolog for the drug-binding site of the enzyme.As with other NNRTIs, etravirine has many drug–drug interactions. It is a substrate of CYP3A4, CYP2C9, and CYP2C19, an inducer of 3A4, and an inhibitor of 2C9 and 2C19. Caution should be used with co-administration of inducers, inhibitors, or substrates of these enzymes. Etravirine can be synthesized starting from 5-bromo-2,4,6-trichloropyrimidine through three successive nucleophilic substitution reactions. Initial displacement with 4-aminobenzonitrile using Hu¨nig’s base, followed by reaction with sodium salt of 4-hydroxy-3,5- dimethylbenzonitrile, and subsequent ammonolysis reaction with ammonia in dioxane under pressure affords etravirine. .
Химические свойства
White to Off-White SolidИспользование
Etravirine is an antiretroviral (anti-HIV) drug that is part of the non nucleoside reverse transcriptase inhibitor (NNRTIs or Non Nukes) family. It is used together with other antiretrovirals in treatment-experienced adult patients, who have failed previous therapy, and have HIV-1 strains which are resistant to other retrovirals and NNRTIs. Etravirine is used to delay the progression of HIV infection. By using etravirine, your immune system should improve (increase in CD4 + count) and you will be better protected against opportunistic infections.Etravirine does not cure AIDS or completely kill the HIV virus, but helps to prevent further damage by slowing down the production of new viruses. Treatment with etravirine does not reduce the risk of passing infection on to others. You will still be able to pass HIV by sexual contact, by blood transfer or by sharing needles. You should always use appropriate precautions to prevent passing HIV on to others.
Определение
ChEBI: An aminopyrimidine that consists of 2,6-diaminopyrimidine bearing a bromo substituent at position 5, a 4-cyano-2,6-dimethylphenoxy substituent at position 4 and having a 4-cyanophenyl substituent attached to the 2-amino group. NNRTI of HIV-1, binds directl to RT and blocks RNA-dependent and DNA-dependent DNA polymerase activitiesПриобретенная устойчивость
Various mutations are associated with a decreased virological response. Single codon substitutions at positions 100, 101 and 181 are considered major mutations. A single K103N mutation is not associated with resistance.Фармацевтические приложения
A comprehensive analysis of baseline resistance data from the DUET-1 and DUET-2 studies has identified a list of 17 etravirine resistance associated mutations: V901, A98G, L100L, K101E/H/I, V1061, E138A, V179D/F/T, Y181C/L/V, G190A/S, and M230L. A single K103N mutation is not associated with resistance to etravirine.Механизм действия
Etravirine binds directly to reverse transcriptase and blocks the RNA-dependent and DNA-dependent DNA polymerase activities by causing a disruption of the enzyme's catalytic site. Etravirine does not inhibit the human DNA polymerases alpha, beta, and gamma.Фармакокине?тика
Oral absorption: Not known/availableCmax 200 mg oral twice daily: c. 959 ng/mL
Cmin 200 mg oral twice daily: c. 469 ng/mL
Plasma half-life: c. 36 h
Volume of distribution: Not known/available
Plasma protein binding: >99%
Administration with food improves the bioavailability and reduces interpatient variability. It undergoes oxidative metabolism by cytochrome P450 systems. Around 93.7% and 1.2% of an administered dose can be retrieved in the feces and urine, respectively, mostly as unchanged drug.
Details of distribution into CSF, semen and breast milk and recommendations for dose adjustment in patients with hepatic impairment are not yet available.
Клиническое использование
Treatment of HIV-1 infection in adults (in combination with other antiretroviral drugs)Побочные эффекты
In the phase III studies around 15% of patients experienced erythematous or maculopapular rashes of mild or moderate severity; most resolved with continued dosing, but treatment was discontinued in 2% of patients. Rare cases of Stevens– Johnson syndrome have been reported.Other common adverse events are diarrhea, nausea, headache and fatigue. Dyslipidemia and raised pancreatic amylase occur in some patients.
Этравирин запасные части и сырье
Этравирин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +8617756083858 | China | 973 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-371-66670886 | China | 19902 | 58 | |
| 010-60279497 | CHINA | 1803 | 55 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +8618957127338 | China | 2136 | 58 |