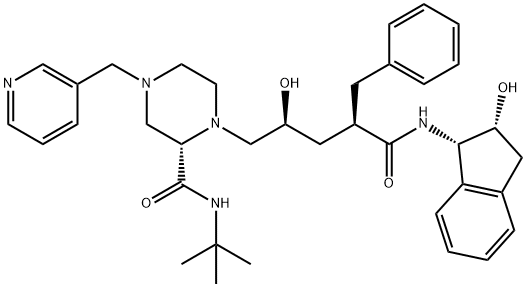
Индинавир
- английское имяIndinavir
- CAS №150378-17-9
- CBNumberCB6123497
- ФормулаC36H47N5O4
- мольный вес613.79
- номер MDLMFCD00866938
- файл Mol150378-17-9.mol
| Температура плавления | 153-154°; mp 167.5-168° |
| альфа | D22 +24.1° (c = 0.0133 in chloroform) |
| Температура кипения | 877.9±65.0 °C(Predicted) |
| плотность | 1.25±0.1 g/cm3(Predicted) |
| температура хранения | Store at -20°C |
| растворимость | Soluble in DMSO |
| пка | pKa 3.8/6.2(H2O,t undefined,Iundefined) (Uncertain) |
| Растворимость в воде | 70mg/L(temperature not stated) |
| FDA UNII | 9MG78X43ZT |
| Словарь онкологических терминов NCI | indinavir |
| Код УВД | J05AE02 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Индинавир химические свойства, назначение, производство
Описание
Crixivan was launched in Australia, Switzerland, the UK and the US as an orally-bioavailable HIV-1 protease inhibitor. The compound can be prepared by coupling an optically active piperazine derivative with an epoxide derivative (now commercially available). The synthesis of the proteins, reverse transcriptase, integrase, structural proteins and a protease, required by the virus to complete its lifecycle, can be interupted if the protease enzyme is not capable of cleaving a proform polypeptide chain into these components. lndinavir inhibits this process and is more potent than the first approved protease inhibitor saquinavir. This effect was noted by the increase in CD4+ cells and a decrease in HIV RNA levels. Since indinavir is metabolized by the CYP3A4 isozyme, care must be taken with patients with hepatic insufficiency and to sex-related differences in the level of this enzyme. Other than nephrolithiasis (5%), indinavir is relatively safe and well tolerated.Показания
Indinavir (Crixivan) is a potent inhibitor of HIV reverse transcriptase. It produces the side effects common to all protease inhibitors and also may produce nephrolithiasis, urolithiasis, and possibly renal insufficiency or renal failure. This problem occurs more frequently in children (approximately 30%) than adults (approximately 10%) and can be minimized by drinking at least 1.5 L of water daily. Additional side effects include asymptomatic hyperbilirubinemia, alopecia, ingrown toenails, and paronychia. Hemolytic anemia rarely occurs. Rifampin should not be given with indinavir.Приобретенная устойчивость
The major mutations in the protease enzyme associated with loss of the antiretroviral activity occur at positions 46, 82 and 84. Generally, the level of resistance rises with the number of point mutations.Общее описание
When administered with a high-fat diet, indinavir(Crixivan) achieves a maximum serum concentration of77% of the administered dose. The drug is 60% bound inthe plasma. It is extensively metabolized by CYP3A4, andseven metabolites have been identified. Oral bioavailabilityis good, with a tmax of 0.8±0.3 hour. The half-life ofelimination is 1.8 hour, and the elimination products aredetectable in feces and urine. Indinavir also causes dyslipidemia.The available dosage forms are capsules of 200 mg,333 mg, and 400 mg.Фармацевтические приложения
A synthetic compound formulated as the sulfate for oral administration.Фармакокине?тика
Oral absorption: c. 65%v Cmax 800 mg thrice daily: c. 8.97 mg/LCmin 800 mg thrice daily: c. 0.15 mg/L
Plasma half-life: c. 2 h
Volume of distribution: c. 0.4–1.74 L/kg
Plasma protein binding: c. 60%
Absorption and distribution
It is rapidly absorbed and not significantly affected by intake with food. Distribution in the body has not been fully characterized. It penetrates well into the CNS. The semen:plasma ratio is 1.9. It is distributed into breast milk.
Metabolism and excretion
Seven major metabolites have been described, including a glucuronide conjugate and six oxidative metabolites. Around 83% of the dose is recovered in feces and 18% in urine, 10% as unchanged drug. The effect of renal impairment has not been studied. It should be used with caution in the presence of hepatic impairment, particularly if severe.
Клиническое использование
Treatment of adult HIV infection (in combination with other antiretroviral drugs)Побочные эффекты
The principal side effect is nephrolithiasis, including flank pain with or without hematuria. There is good evidence that indinavir directly causes nephrolithiasis as a result of crystallization in the urinary tract. Indirect hyperbilirubinemia occurs in about 10% of patients associated with inhibition of bilirubinconjugating activity occurring as a result of competitive inhibition of uridine diphosphate (UDP)-glucuronosyltransferase.Ritonavir-boosted indinavir is associated with a dyslipidemia profile characteristic of those treated with other protease inhibitors boosted with a 200 mg dose of ritonavir per day. Insulin resistance and hyperglycemia have also been associated with ritonavir-boosted indinavir.
Метаболизм
The usual oral dose for indinavir alone or in combination with other antiviral agents is one 800 mg capsule every 8 hours. The drug is well absorbed if given on an empty stomach or 1 hour before or 2 hours after a light meal with water. The dose is reduced to 600 mg every 8 hours if given concurrently with ketoconazole. Indinavir activity is increased when combined with RT inhibitors.Индинавир запасные части и сырье
Индинавир поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 0086-13720134139 | CHINA | 965 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| 0917-3909592 13892490616 |
China | 9310 | 58 | |
| +8615604608665 15604608665 |
CHINA | 9414 | 58 | |
| +8615056975894 | CHINA | 9911 | 58 | |
| 0592-5800732; +8613806035118 |
China | 988 | 58 | |
| China | 12341 | 58 |