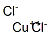
Меди(II) хлорид
- английское имяCopper(II) chloride
- CAS №7447-39-4
- CBNumberCB1144723
- ФормулаCl2Cu
- мольный вес134.45
- EINECS231-210-2
- номер MDLMFCD00010972
- файл Mol7447-39-4.mol
| Температура плавления | 620 °C(lit.) |
| Температура кипения | 993°C/760mmHg |
| плотность | 3.386 g/mL at 25 °C(lit.) |
| Плотность накопления | 1200kg/m3 |
| давление пара | 0Pa at 25℃ |
| температура хранения | Store below +30°C. |
| растворимость | H2O: soluble |
| форма | powder |
| цвет | Yellow-brown |
| Удельный вес | 3.386 |
| РН | 3.5 (50g/l, H2O, 20℃) |
| Растворимость в воде | 620 g/L (20 ºC) |
| Чувствительный | Air Sensitive & Hygroscopic |
| Мерк | 14,2633 |
| Пределы воздействия | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| Стабильность | Stable. Reacts violently with potassium and sodium. Contact with acetylene may form explosive acetylides. Hygroscopic. |
| Surface tension | 72.7mN/m at 1.01g/L and 21℃ |
| Справочник по базе данных CAS | 7447-39-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | P484053J2Y |
| Справочник по химии NIST | Copper chloride(7447-39-4) |
| Система регистрации веществ EPA | Cupric chloride (7447-39-4) |
| UNSPSC Code | 12352302 |
| NACRES | NA.23 |
| Коды опасности | N,T,Xn,Xi | |||||||||
| Заявления о рисках | 52/53-50/53-36/37/38-25-22-51/53-41-23/24/25-50-36-21/22-38 | |||||||||
| Заявления о безопасности | 61-57-45-37/39-29-26-60-36-44-36/37/39-28-39 | |||||||||
| РИДАДР | UN 3264 8/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | GL7000000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28274990 | |||||||||
| Банк данных об опасных веществах | 7447-39-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 584 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H318:При попадании в глаза вызывает необратимые последствия.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H302+H312:Вредно при проглатывании или при попадании на кожу.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Меди(II) хлорид химические свойства, назначение, производство
Химические свойства
Cupric chloride, CuCI2, also known as copper chloride, is a yellowish- brown solid that is soluble in water and alcohol. The dihydrate of cupric chloride, CUCI2·H20, is a green crystalline solid that is soluble in water. Cupric chloride is used in the textile industry as a mordant in the dyeing and printing of fabrics. Itis also used in refining gold,silver,and copper.Использование
Isomerization and cracking catalyst, mordant in dyeing and printing fabrics, in electroplating baths for plating Cu on Al, in photography as a fixer, as a pigment for glass and ceramic.Copper(II) chloride is used as a co-catalyst with palladium(II) chloride used in the Wacker process to prepare ethanol from ethane. It acts as an effective catalyst for the tetrahydropyranylation of alcohols. It is involved in the production of vinyl chloride and dichloroethane as well as in the chlorination of aromatic hydrocarbons. It acts as deodorizing and desulfurizing agent in the petroleum industry, a mordant in textiles industry and in electrotyping baths for plating copper.Определение
ChEBI: An inorganic chloride of copper in which the metal is in the +2 oxidation state.Общее описание
Cupric chloride and ammonia contains various concentrations of cupric chloride in ammonium hydroxide. This forms a copper ammonia complex. Cupric chloride is corrosive to skin, eyes, mucous membranes and metal. Cupric chloride is used to etch copper from printed circuit boards.Профиль реактивности
CUPRIC CHLORIDE has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.Угроза здоровью
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Профиль безопасности
Poison by intravenous and intraperitoneal routes. Experimental reproductive effects. Mutation data reported. See also COPPER COMPOUNDS and CHLORIDES. Can react violently with K and Na. When heated to decomposition it emits toxic fumes of Cl-.Перевозки
UN2802 Copper chloride, Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
Crystallise the chloride from hot dilute aqueous HCl (0.6mL/g) by cooling in a CaCl2-ice bath. It is dehydrated by heating on a steambath under vacuum. It is deliquescent in moist air but efflorescent in dry air. The dihydrate is emerald green but blue when free from solvent. Concentrated solutions are yellow-green in colour but are blue when free from solvent. Concentrated solutions are yellow-green and become yellow on adding conc HCl. A very dilute solution is pure blue due to Cu(H2O)42+ [Donan & Bassett J Chem Soc 81 939 1902.]. CuCl2 is very deliquescent and is soluble in MeOH or EtOH to give green crystals of Cu(ROH)2Cl2. [Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1008 1965.]Несовместимости
Contact with strong acids forms monovalent copper salts and toxic hydrogen chloride gas. Forms shock-sensitive and explosive compounds with potassium, sodium, sodium hypobromite, nitromethane, acetylene. Keep away from moisture and alkali metals. Attacks metals in the presence of moisture. Reacts with moist air to form cupric chloride dihydrate. May attack some metals, paints, and coatings. May be able to ignite combustible materials.Меди(II) хлорид запасные части и сырье
Меди(II) хлорид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-13806087780 | China | 17365 | 58 |